Non Bonded Interactions in a Cylindrical Capacitor of (M, N) @ (M', N') @ (M”, M”) three Walled Nano Carbon Nanotubes
Nabieh Farhami, Majid Monajjemi and Karim Zare
and Karim Zare
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: m_monajjemi@srbiau.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/330640
In this study we have exhibited that there are regular non-bonded interactions among the layers of Three walled Carbon nanotubes (TWCNTs) and (10, 10), (8, 8) DWCNTs & (6,6) SWBNNTs. It has been observed the energies between three layers are sensitive to chirality dramatically. In other hands the external fields of the inner tubes make strongest fields for the outer tubes. It has been shown the non-bonded quantum mechanics in terms of wave functions and charges densities between three layers. For the systems including charges Q the densities (|ψ(x)2|) multiplied the atomic charges, yielding toward the charges densities of (|ψ(x)2|.
KEYWORDS:Non-covalent interaction; three walled Nano tubes; cylindrical capacitor
Download this article as:| Copy the following to cite this article: Farhami N, Monajjemi M, Zare K. Non Bonded Interactions in a Cylindrical Capacitor of (M, N) @ (M', N') @ (M”, M”) three Walled Nano Carbon Nanotubes. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Farhami N, Monajjemi M, Zare K. Non Bonded Interactions in a Cylindrical Capacitor of (M, N) @ (M', N') @ (M”, M”) three Walled Nano Carbon Nanotubes. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40754 |
Introduction
Three walled nanotubes (T-WCNTs) can be considered as a tube twisted from hexagon sheets of graphene or boron nitrides plates which have been synthesized1 in 1995 firstly. Boron nitrides tubes have structural analogs as carbon nano tubes. SWBNNTs, in contrast of CNTs is metallic or semiconductors depends on its chirality1, boron nitrides tube are occasionally believed2 to be a 1-dimension insulators regarding of their helicity3, tube’s diameter and the number of tubes wall4.
Since BNNTs were synthesized1 via arc5 discharges successfully, the mechanism6 for its formations are obscure7 yet, and the behaviors of the metallic catalyst remain an unknown question7.
Several experiment results showed which small CNTs are occasionally found inside three-walled tubes8-12. Therefore, there are strong motivations for studding in phenomenon of their stabilities in small BNNTs inside the largest one12-15, which might be helping the experimental11 synthesis12-14. Besides15, Studding on Three-walled NNTs (TWNTs) has demonstrated14,15 interesting16 variations in their electronics properties17 when they are compared18 with those of freestanding17,18 components . Therefore it is also important for seeing the inter-walled coupling18 behaviors associated with those small BNNTs19,20.
Internal-layers couplings play a critical19 function for determining properties21 of layered three-dimensional22 material. Truly, the novel studies have exhibited which mechanical20 coupling among the outer and inner shells of T-WCNTs leads19,20 for characterizing radials-breathing20 vibration that corresponds to oscillated modes21 with concerted22 inner-and outer-shells motion.
 |
Figure 1:(10,10),(8,8),(6,6) TWCNTs in view point of Cartesian orientation Click here to View figure |
 |
Figure 2: (10, 10), (8, 8)DWCNTs &(6,6) SWBNNTs in view point of Cartesian orientation Click here to View figure |
It has been exhibited which TWCNTs are having high oxidative resistance21, which were later confirmed23, by other scientists. Recently, some results indicate which purified22 SWBNNTs are resist for oxidase up to 1,100°C23. Furthermore, SWBNNTs are predicted to have piezoelectricity24 character and are applicable25 for room-temperatures hydrogen26 storages.
An elastic26 property of an individual SW-BNNT has been clearly studied through thermal vibration27 methods.
SWBNNTs were theoretically predicted28 the uniforms electronics properties29 which are related to its chirality and also diameters2,3.In addition, zigzags chiral are expected28 for having direct28 bands gaps. Other word, the armchairs tubes shall have in directs band28 gaps. Due to their large band gaps using MWBNNTs as the conduction29 channel for fields-effects transistors29 indicated that MWBNNTs allowed transports through the valence29 bands. Important points concerning the bands gap for boron nitrides Nano tube are that those are tunable12,13 via doping15 with carbons. Theoretical band gaps and densities of states calculation in point of view Fermi17-29 level suggested27,28 that SWBNNTs can either be p – types or n – types semiconductor through controlling24-28 the composition33 of carbons into SWBNNTs .
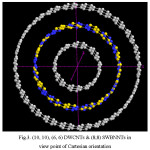 |
Figure 3: (10, 10), (6, 6) DWCNTs & (8,8) SWBNNTs in view point of Cartesian orientation Click here to View figure |
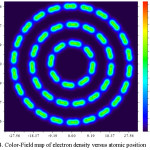 |
Figure 4: Color-Field map of electron density versus atomic position Click here to View figure |
The piezoelectricity approaches originated34 from the deformations effects because of twisting of the h-BN planes for forming the tubes structures. It is an important behavior in BNNTs24. It has been exhibited that BNNT can be amazing piezoelectric24 system while the piezoelectric constants for different zigzags BNNT was defined for increasing along25 with the decreases of the radiuses of BNNTs25. One of the most important features of BNNTs is that BNNTs possess large band gaps between 5.0 and 6.0 electron volte irrespective29 of the number of BNNT’s wall, diameter and chirality2,16-27 .
In this study we have exhibited that there are regular18 non-bonded interactions among the layers of TWCNTs and it has been observed the energies between three layers are sensitive to chirality dramatically12. In other hands the external fields of the inner tubes make strongest fields for the outer tubes.
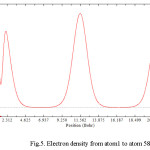 |
Figure 5: Electron density from atom1 to atom 588 Click here to View figure |
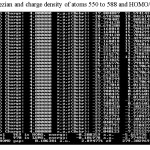 |
Table 1: Cartezian and charge density of atoms 550 to 588 and HOMO/LUMO gaps Click here to View table |
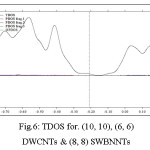 |
Figure 6: TDOS for. (10, 10), (6, 6) DWCNTs & (8, 8) SWBNNTs Click here to View figure |
It has been shown the non-bonded quantum mechanics in terms of wave functions and charges Q the densities (|ψ(x)2|) multiplied the atomic charges, yielding toward the charges densities of |ψ(x)2|. Therefore, there are good relationships among charge and wave functions of those inner tubes rather than outers . Although the wave functions of each molecule in the systems for ΦnInnertube and Φmoutertube under the permutations of electrons coordinate is anti-symmetric, the product of those non-bonded molecule under intermolecular exchanges of the electron could not be anti-symmetric.
The unique solution for this purpose is introducing the intermolecular anti-symmetrize operators. However, these would results due two major problem which were shown by Eisenschitz38 , that are, the anti-symmetrized unperturbed state no longer eigen-function of H(0), which will be followed via the [Ã,H(0)]≠0, hereby the projected excited state, ÃInn-outΦnInn Φmout,become linearly dependent38,39 . and the first-order energies including exchanges could be written as:
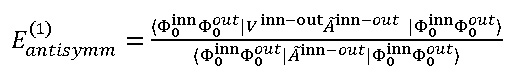
Generally, the exchange interactions are commensurate19 to the differential overlaps among other parts. The wave-function as well as the exchange interactions fades exponentially as functions of distance38,39 .Therefore, TWCNNs with small diameter and short distance between three layers have been considered based on our previous works30-49.
Computational details
Calculations were accomplished by Gammas package50. In this study, we have calculated for getting the result from DFT method and extended-hackles approaches for the no-bonded interactions among tubes. The m06-2x, and m06HF are new method with suitable corresponds in no-bonded interactions and are important for the distance calculations among fragments in the non-bonded51 systems, for medium51 (∼3–5Å) . We investigated density functional theories with theory of van der Waals densities for modeling exchange-correlations energies for the nanotubes3-22. The double ζ-basis set including polarize orbitals was used for boron and nitrogens atoms while single ζ-basis set including polarize orbital was applied for the TWCNTs, respectively53.
For non-covalent interactions, the BLYP methods are unable for describing van der Waals51-53 TWCNTs systems by medium-ranges interaction such as interaction between three tubes of TWCNTs. The B3LYP and some other functional are not suitable for illustrating the exchanges and correlations energies in distant medium-range non-bonded system obviously. In addition, some novel studies have exhibited that inaccuracies of the medium-ranges exchanges energies lead to a systematic error to predict of molecular properties53-58 .
Geometry optimization and electronic structures have been accomplished using the semi empirical methods such as CNDO, MINDO, and ZINDO which their approach are based on an semi empirical solution ab-initio and the Kohn-Sham equation57 also. The density function theories in the plane-waves sets with the projector-augmented wave pseudo-potential are calculated. The Perdew-Burke-Ernzerhof58exchange-correlations functional for the generalized gradient approximations or GGA are adopted58. The optimization of the lattices constant and the atomics coordinate are made through the minimization of those total energies.
The charges transferring and electrostatic potentials-derived were also calculated using the Merz-Kollman-Singh59, chelp60, or chelpG61.
The charges calculations method based on molecular electrostatics potentials fitting are not complete-suited for estimating of bigger systems which some of those inner-most atom are located62 far away from that point at which the MESP62 is computed60-62. In these conditions, variation of the inner-most atomics charge would not leads to a significant change of the MESP61 outside of the molecules, meaning where the accurate62 value for the innermost atomics charge are not well-determined by MESP62 outside63 of those molecules .The representatives atomics charge for molecule must be computed as averages value over various molecules and conformation. A detailed overview of those effect of those basis sets on the charges distribution might be found in63,64 . It has been extracted the charges densities profile from the first-principle calculations through the average process described65,66.
The interaction energies for capacitors were calculated through equation: ΔEs (eV)= {ETWCNT-(ESWCNT(1)+ESWBNNT(2)+ ESWCNT(3))}+ EBSSE where the “ΔEs ” are the stabilities energies of non-bonded interactions in TWCNTs.
Result and Discussion
The results are plotted in 6 curves and HOMO LUMO is listed in Table1.
We have estimated the electron densities and Gradient norms & Laplacians, values of orbitals wave-functions, electron spin densities, electrostatic potentials from nuclear / atomics charge, electron localization functions or ELF64-66, localized orbitals locator or LOL64-66 defined via Becke & Tsirelson, total electrostatics potentials , and the exchange-correlations densities, correlations hole and correlations factor, Averages local ionization energies using the Multifunctional66 Wave-functions Analyzer65,66 Through Density-of-state DOS66 it has been represented the number of state in the units energies intervals, since energies level are contiguous, so DOS66 has drawn as curves map. The systems are isolated, so the energies levels are discrete66.
We have investigated the DOS66 graphs as the tools for analyzing the natures of electron structures in the nanotubes. The original total DOS (TDOS) of our system have calculated based on TDOSE(E) = Σi δ(E-εi) where ε eigenvalue set of single-particle Hamilton is, is Dirac Delta function which after replacing Gaussian, it can be yielded63.
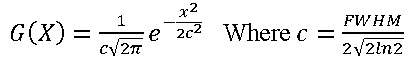
FWHM63 is acronyms of “full width at half maximum63, it is an adjustable parameter in multi-wave function, the larger FWHM the TDOS analysis is easier for performing. The normalized Lorentzian function is defined as L (X) = FWHM/2π 1/x2 +0.25FWHM2 Pseudo-Voigt function is weighted linear combination for Gaussian function and Lorentzian formula: P(x) = wgaussG(x)+(1-Wgauss)L(x).
The curve map of broadened partial DOS (PDOS) and overlap DOS (OPDOS) are valuable for visualizing orbital composition analysis, PDOS function of fragment A is defined as: PDOSA(E) = Σi Ξi,A F (E-εi) where is the composition of fragment “A” in orbital i. The OPDOS among fragments A and B are defined as OPDOSA,B(E)= ΣiXiA,B F (E-εi) where Xi A,B A, B is the composition of total cross term between fragment A and B in orbital i .
Both original and broadened TDOS/PDOS/OPDOS are shown in the graph below. The original DOS graphs are discrete comb-like lines, from which we cannot obtain any additional information other than energies level distributions, it is impossible for distinguishing different types of a line and degenerate energies levels owing for overlapping.
From the height of black curves (TDOS) we can clearly know how dense the energies levels are distributed everywhere. Besides, the curves corresponding to TDOS, PDOS (red line for fragment 1, blue line for fragment 2) and OPDOS (green line) no longer overlap, we can clearly identifies characters of each orbitals through observing those curves67-69.
References
- Chopra, N. G., Luyken, R. J., Cherrey, K., Crespi, V. H., Cohen, M. L., Louie, S. G., and Zettl, A., Science 1995, 269, 966-7
CrossRef - A. Rubio, J. L. Corkill, and M. L. Cohen, Phys. Rev. B , 1994, 49, 5801
- X. Blase, A. Rubio, S. G. Louie, and M. L. Cohen, Europhys. Lett. 1994, 28, 335
CrossRef - L. F. Sun, S. S. Xie, W. Liu, W. Y. Zhou, and Z. Q. Liu, Nature ,2000, 403, 384
CrossRef - X. Zhao, Y. Liu, S. Inoue, T. R. Suzuki, O. Jones, and Y. Ando, Phys. Rev. Lett.2004,92, 125502
CrossRef - L. Guan, K. Suenaga, and S. Iijima, Nano Lett.2008 8, 459
CrossRef - S. Okada, S. Saito, and A. Oshiyama, Phys. Rev. B 65, 2002,165410.
CrossRef - Levshov, D.; Than, T. X.; Arenal, R.; Popov, V. N.; Parret, R.; Paillet, M.; Jourdain, V.; Zahab, A. A.; Michel, T.; Yuzyuk, Y. I., et al., Experimental Evidence of a Mechanical Coupling between Layers in an Individual Double-Walled Carbon Nanotube. Nano Lett. 2011,11, 4800-4804.
CrossRef - Liu, K. H.;Hong, X. P.; Wu, M. H.; Xiao, F. J.; Wang, W. L.; Bai, X. D.; Ager, J. W.; Aloni, S.; Zettl, A.; Wang, E. G., et al., Quantum-Coupled Radial-Breathing Oscillations in Double-Walled Carbon Nanotubes. Nat. Commun. 2013,4, 1375.
CrossRef - D. Golberg , Y. Bando , K. Kurashima and T. Sato , Scripta Mater. 44 (2001) 1561
CrossRef - Y. Chen , J. Zou , S. J. Campbell , and G. L. Caer , Appl. Phys. Lett, 2004, 84 ,2430
CrossRef - C. Y. Zhi , Y. Bando , C. C. Tang , R. G. Xie , T. Sekiguchi and D. Golberg , J. Am. Chem. Soc.2005, 127,15996
CrossRef - E. J. Mele and P. Kral, Phys. Rev. Lett. 2002, 88 ,56803
CrossRef - S. M. Nakhmanson , A. Calzolari , V. Meunier , J. Bernholc and M. B. Nardelli ,2003, Phys. Rev. B 67, 235406 .
CrossRef - S. H. Jhi and Y. K. Kwon, Phys. Rev. B.2004. 69 , 245407
CrossRef - N. G. Chopra and A. Zettl , Solid State Commun, 1998,105 , 297.
CrossRef - A. Loiseau , F. Willaime , N. Demoncy , G. Hug and H. Pascard , Phys. Rev. Lett. 1996, 76 , 4737
CrossRef - M. Radosavljevic , J. Appenzeller , V. Derycke , R. Martel , Ph. Avouris , A. Loiseau , J.-L. Cochon and D. Pigache , Appl. Phys. Lett.2003, 82,4131
CrossRef - Y. Miyamoto, A. Rubio, M. L. Cohen and S. G. Louie, Phys. Rev. B , 1994, 50,4976
CrossRef - E. J. Mele and P. Kral , Phys. Rev. Lett. , 2002, 88 (2002) 56803 .
- S. M. Nakhmanson , A. Calzolari , V. Meunier , J. Bernholc and M. B. Nardelli , Phys. Rev. B ,2003, 67, 235406 .
CrossRef - Blase, X., Rubio, A., Louie, S. G. & Cohen, M. L. Quasiparticle band structure of bulk hexagonal boron nitride and related systems. Phys. Rev. B , 1995, 51, 6868
CrossRef - Xiang, H., Yang, J., Hou, J. & Zhu, Q. First-principles study of small-radius Single-walled BN nanotubes. Phys. Rev. B , 2003, 68, 035427
CrossRef - R. Eisenschitz and F. London, Z. Physik 60, 491(1930) and Z. Physik.Chemie, 1937, 33, 8-26
- O. Hirschfelder, C. F. Curtiss, and R. B. Bird, Molecular Theory of Gases and Liquids, Wiley, New York, 1954
- Monajjemi, M.; Najafpour, J. Fullerenes, Nanotubes, and Carbon Nanostructures, 2014, 22(6): 575–594
CrossRef - P. Pyykkö, C. Wang, M. Straka , and J. Vaara, Phys. Chem. Chem. Phys. 9, 2007, 2954-2958 (2007).
- C. Wang, P. Pyykkö, and M. Straka, Phys. Chem. Chem. Phys. 2010, 12, 6187-6203
CrossRef - Mahdavian, L.; Monajjemi, M.; Mangkorntong, N. Fullerenes, Nanotubes and Carbon Nanostructures, 2009, 17 (5), 484-495
CrossRef - Monajjemi,M.; Nayyer T. Mohammadian J. Comput. Theor. Nanosci. 2015, 12, 4895-4914.
CrossRef - Monajjemi, M.; Lee, V.S.; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F. J. Phys.Chem C. 2010, 114, 15315
CrossRef - Monajjemi, M. Struct Chem. 2012, 23,551–580
CrossRef - Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013, 117, 1670 −1684
CrossRef - Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22 (4),673-692
CrossRef - Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics. 2014, 433, 1-11
- Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
CrossRef - Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
CrossRef - Tahan, A.; Monajjemi, M. Acta Biotheor, 2011, 59, 291–312
CrossRef - Mollaamin, F.; Monajjemi, M. Physics and Chemistry of Liquids .2012, 50, 5, 2012, 596–604
- Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
CrossRef - Monajjemi, M. Theor Chem Acc, 2015, 134:77 DOI 10.1007/s00214-015-1668-9
CrossRef - Monajjemi, M. Journal of Molecular Modeling , 2014, 20, 2507
CrossRef - Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36, 11, 865–
- Monajjemi, M. Biophysical Chemistry. 2015 207,114 –127
CrossRef - Jalilian,H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015, 54, 8, 08510
- Monajjemi, M.; Mollaamin, F. Journal of Computational and Theoretical Nanoscience, 2012, 9 (12) 2208-2214
CrossRef - Mollaamin F, Varmaghani Z, Monajjemi M. 2011; 49: 318-336.
- Mollaamin, F.; Baei, MT.; Monajjemi, M.; Zhiani, R.; Honarparvar, B.;49. Russian Journal of Physical Chemistry A, Focus on Chemistry, 2008, 82 (13), 2354-2361
CrossRef - M Monajjemi, L Mahdavian, F Mollaamin, B Honarparvar,Fullerenes, Nanotubes and Carbon Nanostructures,2010, 18 (1), 45-55
CrossRef - Schmidt MW,Baldridge kk,Boatz, Elbert ST, Gordon Ms, Jensen JH, Koseki S, Matsunaga N,Nguyen KA, J Compt chem, 2004, 14(11)1347
- Yan Zhao, Donald G. Truhlar, Theor Chem Account (2008) 120:215–241
CrossRef - Yan Zhao, Donald G. Truhlar, Accounts of Chemical Research, 2008, 41, 2, 157-167
CrossRef - Check, C. E.; Gilbert, T. M. J. Org. Chem , 2005, 70, 9828–9834
CrossRef - Grimme, S. Seemingly. Angew. Chem., Int. Ed. 2006,45, 4460–4464.
CrossRef - 55.Wodrich, M. D.; Corminboeuf, C.; Schleyer, P. v. R.. Org. Lett. 2006, 8, 3631–3634.
CrossRef - Schreiner, P. R.; Fokin, A. A.; Pascal, R. A., Jr.; de Meijere, A. Org. Lett. 2006, 8, 3635–3638.
CrossRef - W. Kohn and L. J. Sham, Phys. Rev. 1965,140 A 1133-1138
- Perdew J P, Burke K and Ernzerhof, Phys. Rev. Lett. 1996, 77, 3865-3868
CrossRef - B.H. Besler, K.M. Merz, and P.A. Kollman, J. comp. Chem.1990, 11, 431
- L.E. Chirlian and M.M. Francl, J.comp.chem.1987, 8, 894.
- C.M. Brneman and K.B. Wiberg, J. Comp.Chem.1990, 11, 361
CrossRef - F. Martin, H. Zipse, J. Comp. Chem.2005, 26, 97 – 105
CrossRef - Balderchi, A.; Baroni, S.; Resta, R. Phys. Rev. Lett. 1988, 61, 173.
CrossRef - Tian Lu, Feiwu Chen, Sinica, 69, 2011, 2393-2406
- Tian Lu, Feiwu Chen, J. Mol. Graph. Model, 2012, 38, 314-323
CrossRef - Tian Lu, Feiwu Chen, Multiwfn: A Multifunctional Wave-function Analyzer, J.Comp. Chem. 2012, 33, 580-592
CrossRef - Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
CrossRef - Monajjemi, M., Chahkandi, B. Journal of Molecular Structure: THEOCHEM, 2005, 714 (1), 28, 43-
- M Monajjemi, L Mahdavian, F Mollaamin, B Honarparvar,Fullerenes, Nanotubes and Carbon Nanostructures,2010, 18 (1), 45-55.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









