Extraction Characteristic and Degradation of Chlorophyll from Suji Leaves (Pleomele Angustifolia)
Nita Aryanti and Aininu Nafiunisa
and Aininu Nafiunisa
Chemical Engineering, Diponegoro University, JL Prof. H. Soedarto, S.H, Tembalang, Semarang Postal Code 50275 Indonesia, Phone (024) 7460053, 7460055 Fax. (024) 746055.
Corresponding Author E-mail: nita.aryanti@che.undip.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330663
This research investigated the effect of extraction method, the addition of NaHCO3 and degradation rate of the chlorophyll extraction from suji leaves. It was confirmed that the ultrasound-assisted extraction obtained superior result than the maceration. A product having high total chlorophyll was extracted in a shorter time by using the ultrasound-assisted extraction. Based on the degradation rate analysis, it was presented that the chlorophyll degradation reaction follows the first-order kinetic models.
KEYWORDS:Chlorophyll; Degradation; Ultrasound-assisted extraction
Download this article as:| Copy the following to cite this article: Aryanti N, Nafiunisa A. Extraction Characteristic and Degradation of Chlorophyll from Suji Leaves (Pleomele Angustifolia). Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Aryanti N, Nafiunisa A. Extraction Characteristic and Degradation of Chlorophyll from Suji Leaves (Pleomele Angustifolia). Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40554 |
Introduction
Food additives are usually applied to enrich certain food characteristics, such as smell, taste, and color as a consequence of consumer requirements. In Indonesia, the maximum use of synthetic colorant as a food additive is limited as stated in the Regulation No. 37/2013 of Agency of Drug and Food Regulation. Some food colorants are not good for our health due to their toxicity1. Since people are now more concern about their health, they prefer to use natural food additives such as natural food colorant. Some examples of natural food colorant have been listed, and chlorophyll was one compound categorized as natural food colorant2. Chlorophyll has an enormous potential to replace synthetic green dye because of its abundant availability in plants. Chlorophyll and its derivatives have been registered as natural dyes with the code E140 in the Codex Alimentarius Commission of European Union3.
Chlorophyll from plants can be extracted by modern classical methods using a solvent to obtain pigment as a solute from the plants. Some classical extraction methods were maceration, percolation, and reflux. The methods were not efficient because they consume more time and energy, involved more solvent, and may cause some degradation of target molecule4,5. For this reason, an ultrasound-assisted was offered to become more efficient extraction method. The ultrasound-assisted extraction offers shorter extraction times, less solvent consumption, energy and cost saving extraction method4,6,7. In addition, the ultrasound-assisted extraction provides higher yields recovery and extraction rate compare to classical extraction as well as has been widely used to extract bioactive compound and antioxidants8,9,10,11. Regarding of the advantages of the ultrasound-assisted extraction methods, the paper seeks to focus on the comparison of the conventional and ultrasound assisted extraction for chlorophyll extraction. Chlorophyll from plant material is readily degradable under many conditions. Degradation of chlorophyll can lead by simultaneous actions of enzymes, acidic environment, light, and heat12. Degradation fades the green pigment in chlorophyll and turns into a fawn. This will degrade the quality of chlorophyll as a food colorant.
Specifically, the objective of this study is to extract chlorophyll from suji leaves (Pleomele angustifolia) using two different methods, maceration and ultrasound-assisted extraction under a specific condition. The effect of various solute to solvent ratio and various amount of NaHCO3 addition as a stabilizer were investigated. Furthermore, the study on the effect of extraction method to the chlorophyll degradation rate is evaluated by applying the specific kinetic model.
Experimental
Extraction of chlorophyll
In this study, Suji leaves (Pleomele angustifolia) were pretreated by washing, size reduction, and blanching. The clean suji leaves were blanched by using distillate water at a temperature of 100oC for 1 minute. The blanching process was carried out to inhibit the action of enzyme chlorophyllase and inactivate the enzymes involved in the spoilage of fresh vegetables or plants13,14. Then, the blanched suji leaves were chopped using a blender until their size was about 5 mm. The chlorophyll extraction was performed by maceration (ME) for 3 hours and Ultrasound-Assisted Extraction (UAE) for 30 minutes at room temperature (27oC). The solute-solvent ratios were 1:2, 1:5, 1:10, and 1:15 with the addition of NaHCO3 as a stabilizer at concentration of 0%, 3%, and 4% (w/w).
Analysis of Total Chlorophyll and Chlorophyll Degradation
Chlorophyll extract obtained from the extraction process were filtered and then centrifuged at 1000 rpm for 10 minutes. Further, the substance supernatants were collected. One milliliter of centrifuged extract was diluted into 10 ml. The concentration of chlorophyll extract obtained by measuring the absorbance at a wavelength of 663 μm and 645 μm with UV-Vis spectrophotometer. Chlorophyll concentration measurements performed using equation15:
C (mg/L) = (20,31.A645 nm + 8,05.A663 nm)
C is total chlorophyll concentration, A645 is the absorbance at 645 μm, and A663 is the absorbance at 663 μm.
The highest values of total chlorophyll obtained from both extraction condition (both ME and AUE) were further evaluated on their degradation of chlorophyll.
Kinetic of chlorophyll degradation
Analysis of chlorophyll degradation kinetic was conducted by storing the extracts (having the highest chlorophyll values) at temperature of 7o(cooler), 20oC and 30oC (incubator) during a certain time interval. The total Chlorophyll for a certain time was measured by UV-Vis Spectrophotometric method. The calculation of Kinetic constant (k), half-life values (t1/2), and activation energy followed the first-order degradation kinetic models13.
Total chlorophyll data from extracts stored at various temperatures for a certain time was used to determine the kinetics of chlorophyll extract degradation. The obtained data was then plotted to determine the degradation rate of chlorophyll extract. The plotted data followed the first-order kinetics, In Cα/Cα0=-k.t, with average r2 values come near to 1 as compared with the second-order kinetic model, 1/Cα= k.t+1/Cα0.
Result and Discussion
Chlorophyll Extraction by ME and UAE
In this section, a comparative study of the effect of extraction type (ME and UAE) and solute-solvent ratio, as well as NaHCO3 addition on the extraction result, was investigated. The is results reported in Figure 1.
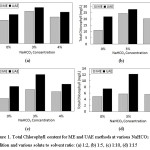 |
Figure 1: Total Chlorophyll content for ME and UAE methods at various NaHCO3 addition and various solute to solvent ratio: (a) 1:2, (b) 1:5, (c) 1:10, (d) 1:15 Click here to View figure |
The figure illustrates that both ME and UAE give the same trend for the different solute to solvent ratio. However, the extraction by UAE method provides better results than the ME method. The best result was obtained at a solute-solvent ratio of 1:2 and 3% addition of NaHCO3 at total chlorophyll value of 41,94 mg/L and 47,03 mg/L for the ME and the UAE, respectively. The similar result was reported for chlorophyll extraction from microalga Nannochloropsis spp. and Chlorella vulgaris16,17 showing a yield increase for the UE compared to the conventional extraction method (ME). The ultrasound-assisted extraction claimed to have superior results than the maceration due to its more efficient process. Moreover, the UAE have some advantages such as less solvent and requirement time to complete the whole extraction process, the higher purity of the final product obtain from UAE process than normally required for a conventional extraction method such as maceration3,9,5. This finding supports the results achieved in this research where the 3-hours ME gave smaller total chlorophyll content of the extract than a 30-minutes UAE. It should be noted that both the ME and the UAE were applied under the same condition.
Furthermore, the UAE gave superior results compared with the percolation, heat reflux, soxhlet extraction and even other new extraction methods such as supercritical fluid extraction and microwave-assisted extraction, when applied to Dunaliella salina and Harpagophytum procumbens4,18. The superiority of UAE methods is mostly due to the effect of ultrasonic waves in the UAE. The waves help the material (mostly natural) to break the cells and release its contents into the solvent as an extraction medium. The ultrasonic wave induces cavitation bubbles around the cell walls, help the cell disruption, and hence there will be a good solvent penetration into the cells6,19.
The addition of NaHCO3 as a stabilizer in both of extraction methods give the same effect. In this research, the highest total chlorophyll was obtained from the extraction with 3% NaHCO3 addition. On the contrary, the extraction without any NaHCO3 addition provides the lowest total chlorophyll. This shows that the addition of NaHCO3 on extraction process can increase the total chlorophyll extracted. Alkaline substances such as NaHCO3 has been used in the blanching process of green vegetables to increase pH and maintain chlorophyll after the process13. The chlorophylls from plants are highly susceptible to degradation during processing. Several factors that affect its stability such as pH, solvent, the intensity of light, enzyme, oxidant, and temperature20,21,22. In general, the degradation of chlorophyll occurs due to the conversion of chlorophyll into its derivative compounds such as pheophytin through pheophytinization which makes the loss of the green color of chlorophyll. In the pheophytinization, Mg atoms in the central core of chlorophyllide will be replaced by two protons to form pheophytin and open the chlorophyll ring structure23,24. The chemical reaction that resulted in the loss of Mg atom from the chlorophyll core ring will be more easily occurs in the acidic conditions. The studies about discoloration and chlorophyll degradation in coleslaw confirmed that the degradation mostly occurs at pH 4.625
Kinetics Reaction of Chlorophyll Degradation
Figure 2 presents the plotted data of time (days) to InCα/Cα0 to determine the degradation rate constant and slope for both extract from maceration and ultrasound-assisted extraction.
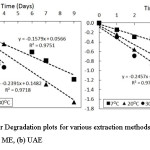 |
Figure 2: First-order Degradation plots for various extraction methods of chlorophyll from suji leaves (a) ME, (b) UAE Click here to View figure |
The figure shows that color degradation on chlorophyll followed the first-order kinetics model. The previous study21,23,26 verified that color degradation on chlorophyll was mostly caused by pheophytinization that lead to loss of green color. The pheophytinization has been said to follow the first-order kinetic model13,27,28.
In addition, it is confirmed that both the extraction methods give similar results of the kinetic model. The kinetic model of chlorophyll degradation from chlorophyll extract by ME and UAE follow the first-order reaction with the increased slope at a higher temperature of storage.
Chlorophyll degradation reaction is a depending-temperature reaction temperature26,29,30. The value of activation energy (Ea) can be determined by using the Arrhenius equation, namely k = A.e-Eα/R. T, this equation linearized then plotted on the graph. This values were calculated on the basis of linear regression analysis of natural logarithms of rate constant against reciprocal absolute temperature (in Kelvin) and displayed in Figure 3.
According to Figure 2 and Figure 3, the degradation rate constant (K), activation energy (Ea), half-life time and other Arrhenius parameters can be calculated. The activation energies values calculated by multiplying the slope of linear regression lines by the R values (1,987 kcal/mol). Table 1 shows this values for the chlorophyll extract from Suji leaves.
Table 1: Degradation and rate constant (k), Arrhenius parameter, and half-life values for chlorophyll extract from Suji leaves
| Extraction Methods | Temperature (Co) | Degradation Rate Constant (k) | Activation Energy (Ea) | Intercept (ln K0) | t1/2 (days) |
| Maceration | 72030 | 0,15790,23910,2848 | 4,3805 | 6,0469 | 4,3892,8992,434 |
| Ultrasound-assisted | 72030 | 0,11630,20280,2457 | 5,5793 | 7,9091 | 5,963,4182,821 |
Figure 2, Figure 3 and Table 1 show that both chlorophyll extract by ME and UAE giving similar trends in different storage temperature. It was observed that the degradation rate of chlorophyll accelerated as the temperature of storage increased. It is also confirmed by the values of half-life (t1/2) on both maceration and ultrasound-assisted extraction that decreased as the storage temperature increase. The rate constant of chlorophyll extract by maceration increased about 1,5 times with increasing storage temperature from 7oC to 30oC. Similar results obtain for the rate constant of chlorophyll extract by ultrasound-assisted extraction which increased about 1,7 times with the same increasing storage temperature. Half life time value for both extract obtained from maceration and ultrasound-assisted method also showed a similar pattern. The degradation reactions need more time to complete at temperature storage 7oC compared to temperature storage of 20oC and 30oC. Chlorophyll degradation reactions occur about 2 times faster at temperatures of 20oC and 30oC in comparison with the storage at temperature 7oC. This result shows that both maceration and ultrasound-assisted extraction will give the similar trends on the reaction of degradation.
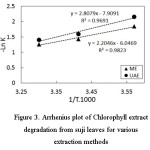 |
Figure 3: Arrhenius plot of Chlorophyll extract degradation from suji leaves for various extraction methods Click here to View figure |
Conclusion
The ultrasound-assisted extraction presented superior result than the maceration method. By using the ultrasound-assisted method, extract having higher total chlorophyll was obtained in a shorter time. The effect of solute to solvent ratio and different NaHCO3 addition provided the same trend for both maceration and ultrasound-assisted method. The optimum value of NaHCO3 addition as a stabilizer for both extraction methods was 3%, confirming the improvement of the total chlorophyll extracted. Analysis of degradation rate by kinetics models verified that the chlorophyll degradation reaction followed the first-order kinetic models. However, the chlorophyll degradation of ultrasound-assisted extraction product had a considerably better ability on the chlorophyll degradation than the product by maceration.
References
- Amchova, P.; Kotolovac, H.; Ruda-Kucerova, J. Regul. Toxicol. Pharm. 2015, 73 (3), 914–922
CrossRef - Mortensen, A. Pure Appl. Chem. 2006, 78 (8), 1477–1491
CrossRef - Baines, D.; Seal, R. Natural food and Beverage Colourings in Natural Food Additives, Ingredients and Flavourings, Published by Woodhead Publishing, Cambridge, 2012, 25-40
CrossRef - Chemat, F.; Rombaut, N.; Sicaire, A. G.; Meullemiestre, A.; Fabiano-Tixier, A. S.; Abert-Vian, M. Ultrason. Sonochem. 2017, 34, 540-560
CrossRef - Baghdikian, B.; Filly, A.; Fabiano-Tixier, A. S.; Petitcolas, E.; Mabrouki, F.; Chemat, F.; and Ollivier, E. C. R. Chim. 2016, 19(6), 692-69
CrossRef - Chemat, F.; Zill-e-Huma; Khan, M. K. Ultrason. Sonochem. 2011, 18, 813-835
CrossRef - Pradal, D.; Vauchel, P.; Decossin, S.; Dhulster, P.; Dimitrov, K. Ultrason. Sonochem. 2016, 32, 137-146
CrossRef - Chen, M.; Zhao, Y.; Yu, S. Food Chem. 2015, 172, 543-550
CrossRef - Yu, X.; Guoyo, T.; Grimi, N.; Bals, O.; Vorobiev, E.; C. R. Chim. 2016, 19, 766-777
CrossRef - Pingret, D.; Fabiano-Tixier, A. S.; Bourvellec, C. L.; Renard, C. M. G. C.; Chemat, F. J. Food Eng. 2012, 111(1), 73-81
CrossRef - Dai, J; Mumper, R. J., Molecules. 2010, 15, 7313-7352
CrossRef - Aparicio-Ruiz, R.; Gandul-Rojas, B. Food Res. Int. 2014, 65, 199-206
CrossRef - Koca, N.; Karadeniz, F.; Burdurlu, H. S. J. Food Chem. 2006, 100, 609-615
CrossRef - Ruiz-Ojeda, L. M.; Penas, F. J. Innov. Food Sci. Emerg. Technol. 2013, 20, 191-197
CrossRef - Zhang, J.; Han, C.; Liu, Z. J. Plant Breed. Crop Sci. 2009, 1(5), 223-229
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F. J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Bioresour. Technol. 2015, 198, 262-267
CrossRef - Kong, W.; Liu, N.; Zhang, J.; Yang, Q.; Hua, S.; Song, H.; Xia, C. J. Food Sci. Technol. 2014, 51(9), 2006–2013
CrossRef - Macías-Sánchez, M. D.; Mantell, C.; Rodríguez, M.; Ossa, E. M.; Lubian, L. M.; Montero, O.; Talanta, 2009, 77, 948-952
CrossRef - Kazemi, M.; Karim, R.; Mirhosseini, H.; Hamid, A. A. Food Chem. 2016, 206, 156-166
CrossRef - King, A.-E.; Liu, C.-F.; Liu, Y.-J. Food Res. Int. 2001, 34, 167-175
- Zhang, X.; Zhang, Z.; Li, J.; Wu, L.; Guo, J.; Ouyang, L.; Xia, Y.; Huang, X.; Pang, X. J. Plant Physiol. 2011, 168, 2081-2087
CrossRef - Jubert, C.; Bailey, G. J. Chromatogr. A. 2007, 1140, 95-100
CrossRef - Hortensteiner, S. Annu. Rev. Plant Biol. 2006, 57, 55-77
CrossRef - Charoenchongsuk, N.; Ikeda, K.; Itai, A.; Oikawa, A.; Murayama, H. Sci. Hort. 2015, 181, 89-94
CrossRef - Boekel, M. A. J. S. V. Food Res. Int. 1999, 32, 261-269
CrossRef - Ngo, T.; Zhao, Y., J. Food Sci. 2007, 72(7), 397-405
CrossRef - Ryan-Stoneham, T.; Tong, C. H., J. Food Chem. Toxicol. 2000, 65(8), 1296-1302
- Gunawan, M. I.; Barringer, S. A., J. Food Process. Preserv. 2000, 24, 253-263
CrossRef - Indrawati, R.; Sukowijoyo, H.; Indriamoko, I.; Wijayanti, R. D. E.; Limantara, L. Procedia Chem. 2015, 14, 353-360
CrossRef - Jaiswal, A. K.; Gupta, S.; Abu-Ghannam, N. J. Food Chem. 2012, 131, 63-72
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









