Corrosion Performance of Stainless Steel and Nickel Alloys in Aqueous Sodium Hydroxide as Revealed from Cyclic Voltammetry and Potentiodynamic Anodic Polarization
M. Abdallah1,2,, M. M. Salem3, I. A. Zaafarany2, A. Fawzy1,4 and A. A. Abdel Fattah1
1Chemistry Department, Faculty of Science, Benha University, Benha, Egypt.
2Chemistry Department., Faculty of Applied Sciences, Umm Al-Qura University, Makkah, Saudi Arabia.
3Chemistry Department, Faculty of Education in Zulfi, Majmaah University, Saudi Arabia.
4Chemistry Department., Faculty of Sciences, Assiut University, Assiut, Egypt.
Corresponding author Email: metwally555@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330621
The electrochemical behavior of nickel, Inconel 600, Incoloy 800 and 316 stainless steel electrodes in different concentration of NaOH solution was investigated using the cyclic voltammetry technique (CV). All the curves in the anodic branch of the CV are characterized by anodic dissolution peak (A),passive region (B),anodic dissolution peak (C) before oxygen evolution. In the case of the cathodic branch of CV there is one reduction peak (D) in case of Ni electrode but there are two reduction peaks (D and E)in case of the three alloys used. All these peaks are explicated. The effect of chloride ion as a pitting agent on the cyclic voltammetry and the potentiodynamic anodic polarization of Ni ,Inconel600,Incoloy800and 316 SS electrodes in NaOH solution was investigated. As the concentrations of the chloride ions increase the change of the amount of the anodic charge Dqa increases and the values of the pitting corrosion potential are shifted to the more negative direction indicating the breakdown of passivity and the imitation of the pitting corrosion. At one and the same Cl- ion concentration, the resistance to the pitting attack decreases in the following order: 316SS > Inconel 600 > Incoloy 800 > 316 SS. This order depends on the chemical composition of these alloys.
KEYWORDS:Nickel; Inconel 600; Incoloy 800; 316 SS; cyclic voltammetry; pitting corros
Download this article as:| Copy the following to cite this article: Abdallah M, Salem M. M, Zaafarany I. A, Fawzy A, Fattah A. A. A. Corrosion Performance of Stainless Steel and Nickel Alloys in Aqueous Sodium Hydroxide as Revealed from Cyclic Voltammetry and Potentiodynamic Anodic Polarization. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Abdallah M, Salem M. M, Zaafarany I. A, Fawzy A, Fattah A. A. A. Corrosion Performance of Stainless Steel and Nickel Alloys in Aqueous Sodium Hydroxide as Revealed from Cyclic Voltammetry and Potentiodynamic Anodic Polarization. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40779 |
Introduction
Nickel alloys and stainless steels (SS) are used extensively in several industrial applications where heat resistance and/or corrosion resistance is required. Nickel is one of the materials most resistant to a variety of caustic solutions (sodium hydroxide), as well as to molten caustic .Various nickel alloys and stainless steel also have been widely used in sodium hydroxide production and in many other industries using caustic applications [1].
The cyclic voltammetry technique is useful for identifying the steps involved in the overall reactions resulting in the formation of various films on the metallic surfaces [2]. It isparticularly useful for distinguishing between products resulting from chemical, electrochemical reactions. Its main limitation is that, it is only useful for examining very thin films formed in a relatively short time.
The characteristic and theory of pitting corrosion has been discussed in details by Kolotyrkin [3] who stated that, the pitting corrosion of a certain metal or alloy occurs within a definite potential range lying in the passive region. The formation and development of this type of attack on the metal surface can occur only in solutions containing the aggressive Cl–, Br– and I– ions[4-12]. The critical potential at which pitting initiates is reported by several authors and found to depend on a number of factors including the type and concentration of attacking anion, type of metal used and relative concentration of other anions[13-14].
In the previous work, [15] the corrosion behavior of nickel, nickel alloys and stainless steel (SS)in various concentrations of HNO3 solutions using the cyclic voltammetry technique was studied. Also the pitting corrosion of these samples in chloride containing solutions and its inhibition by some inorganic compounds using potentiodynamic anodic polarization was also studied [16]. To complement this work,the cyclic voltammetry of Ni, Inconel 600, Incoloy 800 and 316 SS electrodes were traced in NaOH solution as a function of NaOH concentration. Also, the effect of chloride ion as a pitting corrosion agent on the cyclic voltammetry and the potentiodynamic anodic polarization of Ni, Inconel 600, Incoloy 800 and 316 SS electrodes in NaOH solution was further examined.
Experimental
The electrodes used in this study are nickel (Ni) ,Inconel 600, Incoloy 800 and 316 SS. The chemical structures of these electrodes are given in Table 1. A cylindrical rod embedded in Araldite with an exposed surface area to the corrosive medium of 1.70 cm2 for Ni, 1.57 cm2 for the Inconel 600,1.48cm2 for the Incoloy 800 and 1.44 cm2 for the 316SS was used. The electrolytic cell, electrode samples and surface treatment of the electrodes are the same as those described in previous work [15,16].
For the cyclic voltammetry and the potentiodynamic anodic polarization techniques were carried out using a Wenking potentioscan, Type POS-73. The current density-potential curves were recorded on X-Y recorder, Type PL-3. Before carrying out any experiment, the working electrode was precathodized (for 20 minutes) at a potential of -1.5V in order to reduce the pre-immersion oxide film formed at the surface of the electrode.
Table 1: The chemical composition of the alloys used.
| Alloy | Ni% | Fe% | Cr% | Cu% | Si% | P% | S% | Mn | C% | Others % |
| Nickel | 100 | – | – | – | – | – | – | – | – | – |
| Inconel00 | 73.42 | 9.33 | 16.10 | 0.03 | 0.118 | 0.007 | 0.006 | 0.38 | 0.04 | Al 0.28, Ti 0.24, Co 0.049 |
| Incoloy 800 | 33.49 | 44.95 | 19.32 | 0.27 | 0.32 | – | 0.007 | 0.81 | 0.063 | Al 0.39, Ti 0.38 |
| 316 SS | 13.12 | 65.80 | 16.50 | – | 0.50 | 0.03 | 0.002 | 1.63 | 0.028 | Mo 2.35 |
Results and Discussion
Effect of NaOH concentration
The curves of Figs. 1-4 show the effect of various concentrations of NaOH solutions on the cyclic voltammetry (CV) characteristics of Ni ,Inconel 600, Incoloy 800 and 316 SS, respectively, upon the polarization range between -1V to +1V at a scanning rate of 25 mV sec-1 versus the saturated calomel electrode (SCE).
Inspection of the curves of Figs. (1-4) reveals that there is anodic dissolution peak (peak A). As the concentration of NaOH increases, the dissolution current density for peak (A) is increased due to the increase of corrosion rate with increasing the alkali concentration. It is clear from the curves of Figs. (1 and 2) for nickel and Inconel 600 (higher nickel content), that peak (A) appears at nearly the same value of potential (at » – 0.7 V). This peak is thought to correspond to a single electrochemical step, which may be the oxidation of Ni to Ni (OH)2 according to the reaction:
Ni + 2H2O → Ni (OH)2 + 2H+ + 2e– (1)
The equilibrium potential of this reaction according to Pourbaix [17] was found to be:
Ep = 0.11 – 0.0591 pH (2)
giving a redox potential of -0.7V (SCE) in 0.1 M NaOH solution, is in a good agreement with the experimentally reported values for peak (A).
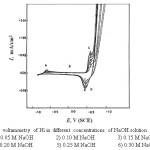 |
Figure 1: Cyclic voltammetry of Ni in different concentrations of NaOH solution at 25 mV/sec. Click here to View figure |
However, there is a sufficient evidence concerning the formation of Ni(OH)2 in alkaline solutions[18] in the potential range corresponding to the anodic current of peak (A). From consideration of the charge amounts under peak (A), several investigators[19,20] gave good evidence for the formation of only a mono-layer of Ni (OH)2 on the electrode surface. It has been reported by Schrebler et al. [21] that Ni (OH)2 appears as hydrated species on the metal surface, once its solubility product is exceeded. Furthermore, in aqueous solutions Ni(OH)2 is bound to a wel1 known equilibrium in solution
Ni (OH)2 = Ni(OH)+ + OH– (3)
Ni (OH)+ = Ni2+ + OH– (4)
and/or Ni(OH)2 = H NiO– + H+ (5)
The latter species are particularly important in alkaline solutions since it entails the anodic dissolution of nickel as a complex anion.
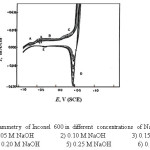 |
Figure 2: Cyclic voltammetry of Inconel 600 in different concentrations of NaOH solution at 25 mV/sec. Click here to View figure |
For Incoloy 800 which contains 33.49% Ni and 44.95% Fe, peak (A) may correspond to the oxidation of Ni and Fe to Ni(OH)2 and Fe(OH)2 respectively. In case of 316 SS (of high iron content) this peak appears at » – 0.6V (S.C.E.), it may correspond to the formation of Fe(OH)2 according to the reaction :
Fe + 2H2O → Fe (OH)2 + 2H+ + 2e– (6)
After peak (A), there is a passive region (region B). The current flowing along this region is commonly identified as the corrosion current which is used to counteract the chemical dissolution of the passive film. The alkali concentration has no significant effect on the current flowing along region (B) of the CV in the case of nickel as shown in Fig. 1, The current remains constant along a wide potential range amounting to about 1V due to the stability of the passivating oxide film. Along this potential region, the passivation current is totally used in repairing the oxide film attacked chemically by the alkali.
Davis and Barker [22] suggested that, the quantity of electricity passed through this region is used in the formation of Ni(OH)2, which fills in any pores existing in the already formed film, the remainder causes a certain amount of the film growth. In case of Ni and Inconel 600, this region may involve the formation of NiO, either by an irreversible chemical reaction:
Ni(OH)2 → NiO + 2H2O (7)
or electrochemical reaction :
Ni(OH)2 → NiO + 2H+ + 2e (8)
X-rays photoelectron spectroscopy (XPS) [23] indicates that the film formed on the Ni electrode surface is probably composed of NiO and Ni(OH)2, the former being the passivating species. The present results are mostly consistent with the spectroscopic conclusions and with the results of Schrebler et al.[21].
The major constituent of the passive film formed on the alloys containing certain amounts of chromium is hydrated chromium oxyhydroxide CrOx(OH)3-2x nH2O [24], where n dependent upon the composition and quality of the underlying alloy and the condition of film formation.
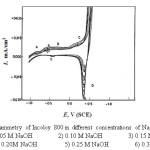 |
Figure 3: Cyclic voltammetry of Incoloy 800 in different concentrations of NaOH solution at 25 Click here to View figure |
As the potential of the electrode becomes more positive, another anodic dissolution peak (C) has been observed before the transpassive region which followed by oxygen evolution. This peak may be attributed to the oxidation Ni (OH)2 or NiO formed at the peak (A) and passive region (B) to some higher oxides of nickel. The oxidation of Ni(OH)2 to a higher nickel oxide [25]. Therefore, in case of Ni and Inconel600, this peak can also be related to the oxidation of Ni (OH)2 to NiOOH according to :
Ni(OH)2 → NiOOH + H+ + e– (9)
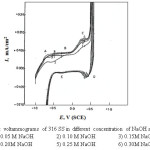 |
Figure 4: Cyclic voltammograms of 316 SS in different concentration of NaOH at 25 mV/sec. Click here to View figure |
Hashimoto and Asami [24] reported that the passive film formed on Inconel 600 consists of Ni(OH)2.nH2O which transforms with further increase in the potential to NiO(OH)2-2ynH2O . In case of Incoloy 800 this peak may be due to the oxidation of Fe(OH)2 and Ni(OH)2 to FeOOH and NiOOH, whereas for 316SS, peak (C) is related to the oxidation of Fe(OH)2 to Fe00H according to:
Fe (OH)2 → FeOOH + H+ + e– (10)
When the potential of working electrode is reversed in the cathodic direction after oxygen evolution, there is one cathodic peak (D) appears in the case of nickel as shown in Fig.1. An additional cathodic peak (E) is observed in case of Inconel600, Incoloy800,316 SS (Figs. 2-4).
In case of nickel the cathodic branch of the CV’s exhibits no further reduction peaks before the evolution of hydrogen is attained. Thus, some of the reduction products of peak (D) probably nickel oxide or oxyhydroxide remains on the electrode surface down to the hydrogen evolution potential. For Inconel 600, Incoloy 800 and 316 SS, this peak can be related to the reduction of the oxyhydroxide formed on the anodic branch of the scan. Peak (E) was related to the reduction of the hydroxide remains in the passive region.
Susceptibility of nickel alloys and stainless steel to pitting corrosion by chloride ions
Cyclic voltammetry measurements
The pitting corrosion of nickel alloys and stainless steel, similar to other metals or alloys, occurs when the passivity breakdown of local points (anodic sites) on the surface exposed to the corrosive medium, whilst the major part remains passive. In practice, the pitting may be encountered if the corrosive environments contain chloride, or other aggressive halide ions [26-28].
The curves of Figs. (5-8) show the cyclic voltammetry of of Ni, Inconel 600, Incoloy 800 and 316 SS respectively, in 0.1 M NaOH solution in the absence and presence of increasing concentrations of NaCl as a pitting corrosion agent at a scanning rate 25 mV/sec.
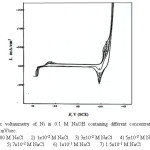 |
Figure 5: Cyclic voltammetry of Ni in 0.1 M NaOH containing different concentration of NaCl solution at 25 mV/sec. Click here to View figure |
 |
Figure 6: Cyclic voltammetry of Inconel 600 in 0.1M NaOH containing different concentration of NaCl solution (at 25 mV/sec.). Click here to View figure |
Inspection of the curves of these figures shows that, the presence of NaCl up to a certain concentration, which depend on the electrolyte bulk concentration and the type of the electrode, is tolerated by the surface film and has practically no effect on the shape of the CVs reported in chloride-free solution. These halide concentrations are assumed to have no influence on the dissolution kinetics of the passive film on the electrode surface. However, higher concentrations of Cl– ions on the other hand, show a tendency to destroy the passive film formed on the electrode surface. The effect of Cl– ions on the passive film formed on the electrode can be recognized by the marked increase of the flowing currents at potentials, in the passive region, which are more active the higher the concentration of the Cl– ions. This increase in current could be attributed to the destruction of the passive film with the initiation of localized corrosion [29-30].
 |
Figure 7: Cyclic voltammetry of Incoloy800 in 0.1M NaOH containing different concentration of NaCl solution at 25 mV/sec. Click here to View figure |
 |
Figure 8: Cyclic voltammetry of 316 SS in 0.1M NaOH containing different concentration of NaCl solution (at 25 mV/sec.). Click here to View figure |
An interesting feature is observed when the potential of the working electrode is reversed in the cathodic direction. The current starts to decrease gradually with decreasing the electrode potential. Finally the current reaches a zero value, then a large dissolution current peak (areas) formed on the cathodic branch of the CVS. These peaks are enlarged with increasing the Cl– ion content of the solution.
The change of the amount of the anodic charge Dqa (coulomb. cm-2), which is the difference between the anodic charge amounts integrated in the presence and absence of Cl– ion, is taken as a measure of the extent of the pitting corrosion to take place [30]. Fig. 9 represents the variation of Dqa with the logarithm of the molar concentration of Cl- ion for Ni, Inconel 600, Incoloy 800 and 316 SS . Dqa changes only slightly in the presence of low Cl– ion concentrations. This may be attributed to repassivation of the formed pits, while at higher concentrations of the Cl– ion, Dqa changes markedly and linearly with log CC1– according to:
Dqa = a1 + b1 log CC1– (11)
where, a1 and b1 are constants which depend on the solution composition and the scanning rate. Under such conditions of Cl– ion concentrations the formed pits may continuously propagate.
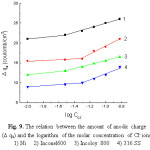 |
Figure 9: The relation between the amount of anodic charge (D qa) and the logarithm of the molar concentration of Cl– ions. |
Potentiodynamic anodic polarization measurements
The curves of Fig.10 represent the potentiodynamic anodic polarization behavior of Ni in 0.1M NaOH solution at a sweep rate of 1.0 mV/sec upon the addition of increasing concentrations of NaCl as a pitting corrosion agent. Similar curves were obtained for the other alloys namely, Inconel 600, Incoloy 800 and 316 SS. Inspection of the curves of Fig. 10 reveals that the following aspects:
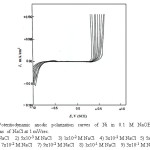 |
Figure 10: Potentiodynamic anodic polarization curves of Ni in 0.1 M NaOH + different concentrations of NaCl at 1 mV/sec. Click here to View figure |
The addition of Cl– ion up to a certain concentration which depends on the OH– ion concentration has practically no effect on the dissolution of the passive film on the metal surface. However, at a certain critical concentration which depends on the alkali concentration, the nature of the metal or alloy, its thermal treatment and the state of its surface. The current flowing along the passive range increases suddenly and markedly at some definite potentials denoting the destruction of the passivating oxide film and the initiation of visible pits. This potential is known the pitting corrosion potential (Epitt) [30-31].
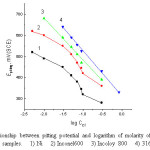 |
Figure 11: The relationship between pitting potential and logarithm of molarity of Cl– ions for nickel and the three alloy samples. 1) Ni 2) Inconel600 3) Incoloy 800 4) 316 SS. Click here to View figure |
The effect of increasing Cl– ion content of the solution is to shift the pitting potential of the working electrode into active (negative) direction. Similar findings were reported by Abdallah.et.al.,[30] in the case of pitting of pitting corrosion of nickel alloys and stainless steel in chloride solutions .
The dependence of pitting corrosion potential of the Ni electrode, Inconel 600, Incoloy 800 and 316 SS on the concentrations of the Cl– ion is shown in Fig. 11 which represents the plot of Epitt versus log CCl- in 0.1M NaOH solutions. Usually most of the investigations carried out on the pitting corrosion potentials reveals a straight-line relationship between Epitting and log CCl– satisfying the following equation
Epitting = a2 – b2 log CCl– (12)
where a1 and b1 are constants which depend on both the nature and the type of the aggressive anion and the electrode sample.
In case of Incoloy 800 and 316 SS straight line relationship are obtained which fit the relationship according to equation (12). In case of nickel and Inconel 600, the curves are segmoid in nature in which equation (12) is obeyed only within a certain range of Cl– ion concentration. At lower of Cl– ion concentrations the pitting potential shift slightly into negative direction as the concentration of the chloride ions is increased. Therefore, one can conclude that these concentrations of Cl– ions are not sufficient to destroy completely the passivating film on the metal surface, or that pits formed are not completely active and may undergo repassivation [32].
The pits formed within the concentration range of Cl– ion, where an equation (12) is applicable are assumed to be of the limiting active type which cannot undergo repassivation. However, at higher concentrations of Cl– ions, The pits formed continuously propagated.
Inspection of Fig.11, it is obvious that, at the same concentration of Cl– ion the noble (positive)shift in the potential decreases in the following order: 316 SS > Incoloy 800 > Inconel 600 > Ni. This sequence is like the previous work in the case of the effect of Cl– ion on the potentiodynamic anodic polarization curves of nickel alloys and 316 SS in HNO3 solution [16]. It is obvious that the 316SS is more resistance to pitting attack due to the presence of Mo and Cr in the chemical composition of 316SS which improve the thickening of the passive film and consequently increase the resistance of this alloy to pitting corrosion. On the other hand, the Incoloy 800 is more resistance than Inconel 600 due to the higher amount of Cr content in its chemical composition..
Conclusions
The cyclic voltammetry technique was constructed of nickel, Inconel600, Incoloy800 and 316 SS electrodes in different concentrations of NaOH solutions.
All the curves in the anodic scan characterized by anodic peak(A), passive region (B), anodic dissolution peak(C) before oxygen evolution.
In the cathodic scan there is is one reduction peak (D) in case of Ni electrode but there are two reduction peaks (D and E)in case of the alloys used.
As the concentrations of Cl– ions increase the change of the amount of the anodic charge Dqa increases and the values of the pitting corrosion potential are shifted to the more negative direction.
At one and the same Cl– ion concentration, the resistance to the pitting corrosion decreases in the following order: 316SS > Inconel 600 > Incoloy 800 > 316 SS.
The resistance of the alloys used to pitting corrosion depends on the chemical composition of its
References
- Crum .J.R., Shoemaker L.E, Corrosion resistance of nickel alloys in caustic solutions, Corros., 2006, 3, 12-16.
- Macdonald. D.P, Cyclic Voltammetry of Copper Metal in Lithium Hydroxide Solution at Elevated Temperatures , J.electochem.Soc.,1974, 121,651-656.
- Kolotyrkin .Y. M., Pitting corrosion of metals, Corrosion,1963, 19 ,261–268.
CrossRef - Abdallah .M, Kamar .E. M, El-Etre A. Y, Salah Eid, Gelatin as corrosion inhibitor for aluminum and aluminum silicon alloys in sodium hydroxide solutions Prot.Met.Phys. Chem. surf., 2016 ,52 (1),140-148.
CrossRef - Abdallah .M, Al Karane S.O., Abdel Fattah A.A, Inhibition of the corrosion of nickel and its alloys by natural clove oil, Chem. Eng.Comm. 2009,196, 1406-1416
CrossRef - Abdallah .M, Al Karane S.O., Abdel Fattah A.A, Inhibition of the corrosion of nickel by natural black cumin oil, Chem.Eng. Comm., 2010,197,1446-1454.
CrossRef - Abdallah .M, Zaafarany.I, Khairou.K. S.,Emad. Y, Natural Oils as Corrosion Inhibitors for Stainless Steel in Sodium Hydroxide Solutions , Chem Tech. Fuels Oils, 2012,48(3), 234-245.
CrossRef - Burstein. G. T., Liu C., Souto R. M, Vines S. P,Origins of pitting corrosion ,Corrosion Engineering, Science and Technology 2004,39., 25-30.
CrossRef - Abdallah .M, Zaafarany. I, Khairou K. S, Sobhi M., Inhibition of carbon steel corrosion by iron(III) and imidazole in sulfuric acid ,Int. J. Electrochem Soc., 2012,7(2),1564-1579.
- Abdallah.M, Asghar B. H., Zaafarany I., Sobhi M., Synthesis of some aromatic nitro compounds and its applications as inhibitors for corrosion of carbon steel in hydrochloric acid solution Prot.Metals. Phys. Chem .surface, 2013,49(4),485–491.
CrossRef - Abdallah .M, El-Etre A.Y., Soliman, M. G ,Some organic and inorganic compounds as corrosion inhibitors for dissolution of C-Stee in 3.5 % NaCl solution. Anti Corros Methods and Materials, 2006, 53 (2),118-123.
CrossRef - AL Jahdaly. B. A., Althagafi I. I., Abdallah .M, Khairou K. S., Ahmed. S. A. ,Fluorenone Hydrazone Derivatives as efficient Inhibitiors of the Acidic and Pitting Corrosion of Carbon Steel. J. Mater. Environ. Sci., 2016 ,7(5),1798-1809.
- Abdallah .M ,AL Jahdaly. B. A., Al-Malyo O. A.,Corrosion Inhibition of Carbon Steel in Hydrochloric Acid Solution using Non- Ionic Surfactants Derived from Phenol Compounds, Int.J. Electrochem Sci., 2015,10 ,2740-2754.
- Jabir H Al-Fahemia, Abdallah .M,,Elshafie A. M. Gad, Jahdaly. B. A., Experimental and theoritcal approach studies for melatonin drug as safely corrosion inhibitors for carbon steel using DFT, J Molecular Liquids, 2016,222,1157-1163
CrossRef - Abdallah .M,Al. Jahdaly. B. A,Salem .M.M, Fawzy.A, Mabrouk E.M., Electrochemical behavior of Nickel Alloys and Stainless Steel in HNO3 using Cyclic Voltammetry Technique. J. Mater. Environ. Sci. 2017, 8(4), 1320-1327.
- Abdallah .M,Al Jahdaly. B. A,Salem .M.M, Fawzy.A, Abdel Fattah A. A.,Pitting Corrosion of Nickel Alloys and Stainless Steel in Chloride Solutions and its Inhibition Using Some Inorganic Compounds , J. Mater. Environ. Sci. 2017,8(7),2599-2607.
- Pourbaix M., Atlas of electrochemical equilibria in aqueous solutions. 2d English ed. 1974, Houston, Tex.: National Association of Corrosion Engineers.
- MacArthur D. M.,The Hydrated Nickel Hydroxide Electrode Potential Sweep Experiments ,J. Electrochem. Soc. 1970., 117, 422-426.
CrossRef - Visscher W., Barendrecht .E., The anodic oxidation of nickel in alkaline solution , Electrochim.Acta., 1980, 25, 651-655.
CrossRef - Medway S.L.,Lucas C.A,Kowal, A,Nichols, R.J,Johnson .D.,In situ studies of the oxidation of nickel electrodes in alkaline solution , J. Electroanal. Chem. 2006, 587 , 173-181.
- Schrebler R.S., Vilche J.R., Arvia A.j, The kinetics and mechanism of the nickel electrode—III. The potentiodynamic response of nickel electrodes in alkaline solutions in the potential region of Ni(OH)2 formation ,Corros.Sci., 1978, 18 ,765-778.
CrossRef - Davies D. E. , Barker W. , Influence of pH on corrosion and passivation of nickel, Corros. ,1964,20, 47 -53 .
CrossRef - Grosvenor A.P.,Biesinger M.C.,Smart R C.,Stewart McIntyre N. , New interpretations of XPS spectra of nickel metal and oxides, Surface Sci,2006, 600,1771-1779.
CrossRef - Hashimoto. H., Asami K., XPS study of surface film on nickel alloys in hot concentrated NaOH, Corros.Sci.,1979,19,427-435.
CrossRef - Fu X.Z., Zhu Y.J.,Xu. Q.C.,Li J., Pan J.H.,Xu J.Q.,.Lin J.D,,Liao D.W. Nickel oxyhydroxides with various oxidation states prepared by chemical oxidation of spherical β-Ni(OH)2 , Solid State Ionics, 2007,178,987-993.
CrossRef - Abdallah ,M, Al. Jahdaly. B. A, Sobhi .M., Ali A.I. ,Evaluation of some nonionic surfactants derived from hydroquinol compounds as corrosion inhibitors for Carbon steel in hydrochloric acid, Int.J.Electrochem Sci,2015, 10(6), 4482-4494.
- Abd ElAal E.E., AbdEl Wanees S.M., Galvanostatic study of the breakdown of Zn passivity by sulphate anions, Corros.Sci.,2003,51,1780-1788.
CrossRef - Abdallah .M, El Etre A. Y ,Mead. A.I., Effect of some nonionic surfactant on the passivity breakdown of Cu-Ni alloys in alkaline chloride solution. J. Electrochemical Soc. of India, 1996,45 (2), 71-78.
- Abdallah .M, Megahed .H. E., El-Naggar M., Radwan D. Mabrouk,E. M. Pitting corrosion of nickel alloys and its inhibition by some dihydrazide derivatives ,Bull Electrochem.,2003, 19(6), 245-254.
- Abdallah .M, Abd El-Haleem S.M., Initiation and inhibition of pitting corrosion of Incoloy 800 and 316 Stainless Steel, Bull. Electrochem., 1996,12 (7-8), 449-458.
- Abdallah .M, Zaafarany I. A, Abd El Wanees S, Assi R., Breakdown of passivity of nickel electrode in sulphuric acid and its inhibition by pyridnone derivatives using the galvanostatic polarization technique , Int.J.Corro. Scale Inib., 2015,4(4) ,338-352
CrossRef - Abdallah .M, Mead. A. I,Pitting corrosion of an iron electrode in HCO–3/Cl– solutions and its inhibition by some nonionic surfactants, Annali Di Chimica: 1993,83,4,24-430.

This work is licensed under a Creative Commons Attribution 4.0 International License.









