Atmospheric Products Microstructure of Grade 3 and Grade 45 Steel Metal Corrosion
Kulash K. Syrmanova 1,2, Zhanat B. Kaldybekova1, Moldir T. Suleimenova2, Yersultan T. Botashev1, Dilyarom A. Abzalova1 and Sholpan B. Baizhanova1
1Auezov South Kazakhstan State University, Kazakhstan, 160012, Shymkent, Tauke Khan av., 5
2 MIRAS” University, Kazakhstan, 160012, Shymkent, Ilyaev st., 5
Corresponding author Email: syrmanova.kulash@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/330664
Article Received on :
Article Accepted on :
Article Published : 08 Dec 2017
This study presents the results of the research devoted to the determination of atmospheric corrosion products of Grade 3 and 45 steel microstructures. By the method of electronic microscopy, a microstructure of internal layers rust after corrosion tests in atmospheric conditions was defined and described. It was found out that the rust has a relatively loose and porous structure, the porosity decreases with increasing depth of the layer and exposure time; the rust layer formed on the underside of the steels is always more porous, roughened and loose than the top; alloying elements, for example, chromium stimulates the formation of a dense and durable layer of rust possessing protective properties. Carried out research allowed to develop the most effective methods of corrosion protection of oil and gas pipeline.
KEYWORDS:corrosion; microstructure; rust; protection; layer; microscopy; porous structure; pipeline
Download this article as:| Copy the following to cite this article: Syrmanova K. K, Kaldybekova Z. B, Suleimenova M. T, Botashev Y. T, Abzalova D. A, Baizhanova S. B. Atmospheric Products Microstructure of Grade 3 and Grade 45 Steel Metal Corrosion. Orient J Chem 2017;33(6). |
| Copy the following to cite this URL: Syrmanova K. K, Kaldybekova Z. B, Suleimenova M. T, Botashev Y. T, Abzalova D. A, Baizhanova S. B. Atmospheric Products Microstructure of Grade 3 and Grade 45 Steel Metal Corrosion. Orient J Chem 2017;33(6). Available from: http://www.orientjchem.org/?p=40745 |
Introduction
The economic losses connected with the corrosion of metals are determined not so much by the cost of the corroded metal as by the cost of repair work, by losses due to the temporary cessation of the functioning of engineering systems, by the costs of preventing accidents, in some cases absolutely unacceptable from the point of view of ecological safety. Estimates of costs associated with corrosion lead to the conclusion that the total annual cost of prevention of the effects of corrosion is 1.5-2% of the gross national product. Part of these costs is inevitable; it would be unrealistic to completely exclude all corrosive fractures. Nevertheless, it is possible to significantly reduce the corrosion losses due to better use in practice of accumulated knowledge of corrosion processes and methods of corrosion protection that anticorrosive services have at the moment.
The term “metal corrosion” includes a big group of chemical processes, leading to a metal destruction. These processes extremely differ from each other in external manifestations, according to the conditions and media in which they occur, as well as the properties of the reacting metals and the reaction products formed. However, for their unification there is a foundation; despite the sharp differences, all these processes have not only a general result – the destruction of metal, but also a single chemical entity – the oxidation of the metal. The thermodynamic instability of metals is the cause of corrosion, as a result of which most of them occur naturally in the oxidized state (oxides, sulfides, silicates, aluminates, sulfates, etc.). Thus, corrosion can be defined as a spontaneous process occurring during the interaction of metal with the environment, accompanied by a decrease in the free energy of Gibbs and the destruction of the metal.
Corrosion proceeds at the interface between the two phases “metal-surrounding medium”, that is, it is a heterogeneous multistage process and consists of at least three main multiply repeating stages [1-2]:
- supply of reacting substances (including corrosive agent) to the phase interface;
- reaction of metal interaction with a corrosive medium, the result of which is the transition of a certain amount of metal into an oxidized form with the formation of corrosion products, and the corrosive agent into a reduced form;
- removal of corrosion products from the reaction zone.
Pipelines used for the transport of oil and gas, in particular commercial and major pipelines, are among the most metal-intensive structures in the oil and gas industry, due to their considerable length, as well as the large diameter of the pipes in the main pipelines and the considerable thickness of the pipe wall in the field pipelines. Most oil and gas pipelines belong to underground structures. In the process of operation, their outer surface is subjected to intense corrosion.
In Kazakhstan domestic oil and gas industry and abroad for anticorrosion protection of the outer surface of oil and gas pipelines, coatings of various varnish-and-paint and polymer materials are used in combination with electrochemical protection for main pipelines or without it for field pipelines. The reliability of the oil and gas pipeline is largely dependent on the outer coating.
Features of the composition and properties of rust in various conditions of the operation often are not taken into account during carrying out anticorrosive operations, in particular at choosing the method of surface preparation. The metal corrosion products formed in different operating conditions have a different phase and chemical composition. Corrosion products formed on the surface of metals can make it slower or accelerate the corrosion process, it depends on their chemical composition, structure and adhesion [3-5]. Therefore, the research of the structure and composition of corrosion products is of practical interest in determining ways to increase the corrosion resistance of metals.
Material and Method
The phase composition of the corrosion products of metals was studied by the radiographic method [6].
To investigate the microstructure of the layers of corrosion products of the profile composition, a scanning electron microscopy method with an attachment for local X-ray spectrometry analysis was used [7].
As an object of the research steel of the grade 3 and 45 were used.
Results and Discussion
There are many researches devoted to the identification of the chemical compounds that are included in the corrosion products of carbonaceous low-alloy steels [8-10]. But there are relatively few studies of the structure of corrosion products on metals, depending on the aggressiveness of the environment. Results of the investigation of the influence of an aggressive medium on the phase composition and microstructure of corrosion products formed on steels grade 3 and 45 was carried out in pipeline operation conditions of PetroKazakhstan Oil Products.
The morphology of corrosion products was studied on transverse sections of corroded samples using the scanning electron microscopy method [7].
Figures 1 and 2 show photomicrographs of the cross sections of rust layers formed on the surfaces of steels grade 3 and 45. Comparison of microstructures of corrosion products of steels grade 3 and 45 showed that rust has a relatively loose and porous structure, consists of fine dispersed iron oxide particles of different sizes, and there is mostly no clearly defined stratification in the cross section of the rust. The inner part of the rust layers on the lower side of the sample facing the ground looks more loose and porous than on the upper side.
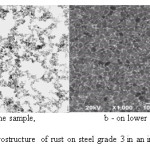 |
Figure 1: Microstructure of rust on steel grade 3 in an industrial atmosphere Click here to View figure |
 |
Figure 2: Microstructure of rust on steel grade 45 in an industrial atmosphere Click here to View figure |
The average thickness of the rust layer varies from 40 to 110 μm. The porosity of the layer decreases with increasing depth of the layer, as well as an increase in exposure. Reducing of the porosity and looseness of the rust layers with the increase of exploitation interval occurs faster on the upper side of the sample than on the lower side. In rust, a relatively large number of cracks and pores are observed, which are a result of elastic strains occurring with the growth of the rust layer. These cracks are usually located more or less parallel to the corroding surface of the steel and are concentrated in the outer part of the layer.
It is interesting that the rust layer contains voids, located between the inner part of the rust layer and the non-corroded surface of the steel. This is especially representative for the steel grade 3. The appearance of such voids in rust can be explained by the unequal expansion of metals and adjacent rust layer under the influence of the air temperature gradient.
As can be seen from the photographs, the rust layer on the lower side of the steel sample is always more porous and rougher than rust of the upper side.
This is due to the fact that on the upper side of the samples steel usually corrodes under permanent water films.
Under the influence of sunlight, which warms the metal surface, the colloidal iron hydroxide particles dehydrate, compact gradually while drying out. On the lower side of the samples, colloidal iron hydroxide is usually formed in individual droplets of water forming from the condensation of atmospheric moisture, mainly at night hours. In the absence of direct solar radiation, this colloid dries out and dehydrates more slowly. The gradual evaporation of water from the inner zones in the rust and the slow dehydration of iron hydroxide colloid are the main reason for obtaining a porous and loose rust film under these conditions.
In industrial conditions, in the presence of C1 ions, deeper local corrosion occurs, and the rust layer has significantly more pores and cracks than rust formed in atmospheric urban conditions. This is apparently due to the fact that the ions of chloride contribute to the active dissolution of the layer of steels corrosion products and the destruction of their structure.
Unlike chloride ions, sulfurous gas positively affects the microstructure of the rust layer. The rust layer on the surface of carbon steel is denser than the one formed in urban conditions. A feature of the rust formed in the industrial atmosphere is the presence of dust particles in the outer part of the layer and the almost complete absence of pores in the inner part. This circumstance apparently also affected the rapid decrease in the rate of corrosion of steel in the industrial atmosphere.
Corrosion products on steel grade 45 in comparison with corrosion products on steel grade 3 have a dark brown color, their granularity is more subtle, the size and quantity of scaly-like aggregates is smaller, the layer is spatially more dense and solid. These differences can be clearer observed with the increasing of atmosphere aggressiveness.
The mechanism for the growth of the rust layer is the following: the outer part of the rust layer which contacts with the air, and also zones around the cracks, pores and very thin boundary lines between the rust and the uncorrodable surface of the steels have a lighter shade compared to other parts of the rust layers. (ПРЕДЛОЖЕНИЕ НЕ ИМЕЕТ СМЫСЛА НА РУССКОМ). With the duration of pores exposure increase the cracks existing on the outer surface of rust, inside the rust or between rust and metal gradually disappear. This indicates that fresh rust can form in all the above places.
It can be assumed that the iron ions formed in the result of the anodic salt dissolution reaction tend to diffuse outward through the rust layer into some zone where the cathodic reduction reaction of oxygen most easily occurs, for example, in a film of water saturated with oxygen. In these zones iron ions react with a hydroxyl group and oxygen forming fresh rust. According to this assumption, when there is a renewable or drying water film on the rust surface, the formation of fresh rust occurs predominantly on the outer surface of the layer of corrosion products contacting with air, at the same time, if there is a sufficient amount of electrolyte impregnating rust, the new rust can form inside cracks and pores in the layer, contributing to the compaction of corrosion products.
With the drying out of deep zones of the layer, the fresh rust gradually starts forming closer to metal surface. This contributes to the elimination of emerging voids between the inner part of the rust layers and the non-corroded steel surface.
Conclusion
The microstructure of the inner layers of rust after corrosion tests under atmospheric conditions has been studied by scanning electron microscopy. Studies showed:
rust has a relatively loose and porous structure, the porosity decreases with increasing depth of the layer and exposure time;
the rust layer formed on the lower side of the steels is always more porous, rough and loose than the upper one;
alloying elements, for example, chromium stimulates the formation of a dense and solid layer of rust possessing protective properties;
chloride-ions contribute to the destruction of the rust structure, sulfuric gas has a favorable effect on its compaction.
References
- Phomin, G.S. Corrosion and protection. International Standarts handbook. Moscow: Protector. 2013, 720
- Decree of the Government of the Republic of Kazakhstan. About the Strategic Plan of the Ministry of Oil and Gas of the Republic of Kazakhstan for 2011-2015. Astana. 2011, 134
- Zhuk, N.P. Course of the theory of corrosion and protection of metals. Metallurgy. 2006, 346
- Rosefield, I.L.; Rubinshtein, F.I. Anticorrosion primers and inhibited paintwork. Chemistry. 1980, 420
- Rosefield, I.L.; Rubinshtein, F.I. Protection of metals from corrosion by paint and varnish coatings. Chemistry. 1987, 255
- Gorelik, S.S.; Rastorguyev L.N.; Skakov, Y.A. X-ray and electron-optical analysis. Metallurgy. 1970, 366
- Myrzakhodzha, D.A.; Mirzakhodzhayev A.A. Modern research methods. Almaty. 2006, 306
- Grosby, N.; Davy. J.; Hardcastle W. Quality in the Analytical Chemistry Laboratory. ACOL. 2005, 21
- Yegorov, O.I.; Chigarkina O.A.; Baimukanov, A.S. Oil and gas complex of Kazakhstan: Development problem and efficiency of functioning. Almaty. 2003, 536
- Syrmanova, K.K.; El-Sayed M. Negim, Tuleuov, A. M., Erkebayeva, G.Sh. The development of composite polymer materials for anti-corrosion protection of oil and gas pipelines. Proceedings of international conference «Application of Efficient & Renewable Energy Technologies in Low Cost Buildings and Construction». Turkey. 2013, 486-490.

This work is licensed under a Creative Commons Attribution 4.0 International License.









