Synthesis and Characterization of Substituted Metal (II) – Octa Methoxyphenyl Imino Phthalocyanine Pigments
Fasiulla and M. P. Yashoda
Department of Chemistry, Manipal Institute of Technology, Manipal, Udupi district, Karnataka, India.
Corresponding Author E-mail: fasiulla1976@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330548
The synthesis, spectral and magnetic susceptibility on symmetrically substituted metal (II) – octa-methoxyphenyl imino phthalocyanine by condensing octa amino phthalocyanines with o-anisaldehyde are described. The dark green colour octa amino phthalocyanine derivatives were characterized by electronic, IR, elemental analysis, magnetic susceptibility, powder X-ray diffraction and thermo gravimetric analysis (TGA) studies to structural integrity, check the purity and crystalline properties of the pigments. The variation of magnetic moment as function of field strength 2.20 to 4.01 kG indicated the presence of intermolecular co-operative effect.
KEYWORDS:Octa methoxy imino phathalocynaine; Electronic; IR; XRD; Magnetic susceptibility; TGA
Download this article as:| Copy the following to cite this article: Fasiulla F, Yashoda M. P. Synthesis and Characterization of Substituted Metal (II) – Octa Methoxyphenyl Imino Phthalocyanine Pigments. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Fasiulla F, Yashoda M. P. Synthesis and Characterization of Substituted Metal (II) – Octa Methoxyphenyl Imino Phthalocyanine Pigments. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=36667 |
Introduction
Phthalocyanines ( Pc) have been worldwide focused on attention to explore and exploit the various versatile chromophores. Phthalocyanines are blue or green in color have been widely exploited as colorants. Phthalocyanines have a strong optical absorbance in the red and near-IR portions of the electromagnetic spectrum and are stable under a wide range of thermal and electrochemical conditions. Besides the optical properties and stability of phthalocyanines, these dyes must be evaluated with regard to their electrochemical suitability and structural suitability for a given application such as an electronic material.
Phthalocyanines like high tinctorial property for use as pigments for printing inks, plastics, rubbers, roofing granules, leather, gasoline and photographic colour printing, fastness to the light, chemical inertness etc. The electro-optic behavior has led to their use in xerography. Phthalocyanines have also been investigated for potential use in a variety of other applications including nonlinear optical generation and limiting, photodynamic therapy, photovoltaics, sensors, electrochromic displays and information storage. Solubility and various physico-chemical properties associated with metal phthalocyanines primarily depend upon the central metal atom and nature as well as the position of the periphery of the molecule.
In the present paper discusses the synthesis and spectral and magnetic susceptibility on substituted metal (II) 1, 3, 8, 10, 15, 17, 22, 24- ocat methoxyphenyl imino phthalocyanines. The literature survey revealed about the reports on synthesis spectral and magnetic susceptibility on substituted (II) octaamino phthalocyanines. The available procedure in the literature was suitably modified and used for the synthetic route. The substituted metal (II) octa-methoxyphenylimino phthalocyanines pigments is given as per the scheme-1.
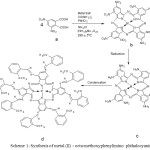 |
Scheme 1: Synthesis of metal (II) – octa methoxyphenylimino phthalocyanine. |
a. 3, 5-dinitrophthalic acid, b. M-PcON c. M-PcOA and d. M-MePhImPcO
The elemental analysis Carbon, Hydrogen, and Nitrogen were performed at Cochin University, Sophisticated Test & Instrumentation Center, Kochi, Kerala, India. The metal content was determined by incinerating them to the oxides. The magnetic susceptibility is carried out at room temperature (301 0K) by using a Gouy balance consisting of NP-53 type electromagnets with a direct current power supply unit and a digital semi-micro balance. The diamagnetic corrections to be calculate using Pascal’s constant.The calibrant mercury tetra thiocyanato cobalt (II) complex was used. Shimadzu UV-Vis spectra recorded spectrophotometer. Infrared spectra were measured using Nicolet MX-FTIR spectrometer with KBr pellets. Philips analytical PW-1710 X-ray diffractrometer was used to study the diffraction pattern. The spectra were recorded using Cu Ka as target material, a current of 20mA, at the voltage of 40 kV, a channel width of 7mm, A time constant of 4, and chart speed of 10mm/min. Thermo-gravimetric analyses were carried out by using a Shimadzu DTG/DTA-60. The TGA and DTA is thermal analyzer at a heating rate of 100C/min in a nitrogen atmosphere and air.
Experimental
The 3, 5-dintrophthalic acid was prepared as reported elsewhere. All necessary chemicals used in the experiment were of analytical grade obtained from commercial supplier. The substituted metal (II) 1, 3, 8, 10, 15, 17, 22, 24-octa amino phthalocyanines complexes prepared by the reported procedure. These octaamino phthalocyanines complexes converted into imino phthalocyanines complexes by using 2-methoxy aldehyde compounds with suitable modification as per scheme-1
Preparation of Copper (II) Octa- Methoxyphenylimino Phthalocyanines Complex
Copper (II), 1, 3, 8, 10, 15, 17, 22, 24- octa-nitro phthalocyanine complex was synthesized according to reported elsewhere. The nitro derivative of the aforesaid complex was converted into amino derivative quantitatively by reduction using sodium sulphide nonahydrate (Na2S 9H2O) in aqueous medium. The finely grounded metal (II) -octaaminophthalocyanine (6.30g / 0.1mole) was added in stiochiometric quantity of 15 M sulphuric acid, to this (13.6g / 0.1mole) 2-methoxy benzaldehyde is dissolved in ethyl alcohol and catalytic amount of con.H2SO4 was added, and the contents were refluxed with stirring for about 5 hrs.
The settled green solid condensed octa- methoxyphenylimino phthalocyanine complex was washed alternatively twice with alcohol free from aldehyde and finally it was washed with distilled water. The complexes dried at 1000C for 40 min and finally in a vacuum over anhydrous phosphorous pentoxide. CoMePhlmPcO, NiMePhlmPcO, ZnMePhlmPcO, were synthesized similarly by using the respective substituted metal aminophthalocyanines.
Results and Discussion
The synthesis of M-MePhImPcO yield pure complexes and it shows that dark green in color except for that of zinc, green with brown tinge. These complexes are sparingly in Dimethyl sulfoxide, Dimethylformamide, pyridine and completely soluble in con.H2SO4. The elemental analysis results are given in Table.1 and the calculated values and are consistent with the suggested structure given below in Fig.1. The reduction of nitro derivatives to the corresponding amino derivatives and the conversion of later into M-MePhImPcO’s were almost quantitative with good yield.
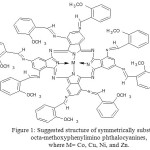 |
Figure 1: Suggested structure of symmetrically substituted octa-methoxyphenylimino phthalocyanines, where M= Co, Cu, Ni, and Zn. Click here to View figure |
Electronic Spectra
The synthesized complexes were recorded DMSO in the concentration range of 1.00 -1.40 X 10-4 M and data are summarized in Table 2. All the absorption peaks are interpreted in terms of Q- band, B-band, L-band and C-band characteristic of phthalocyanine molecule. M-Me Phlm PcO complexes a peak were observed in the wavelength 726-749 nm, which was assigned to Q-band, a1u → eg transition and 327-337 nm assigned to B-band due to a2u → eg transition. The Q-band is due to M-Me PhIm PcO’s was observed to be shifted to higher wavelength range. The peaks are observed in the wavelength range 577-593 nm for Cu-Me PhIm PcO, Co-Me PhIm PcO, Ni-Me PhIm PcO, and Zn-Me PhIm PcO which is accounted for aggregation of complexes in the solvent. For all M-Me PhIm PcO’s a band was observed in the wavelength range of 214-226nm suggested C-band of the phthalocyanine complexes.
Infrared Absorption Spectra
Infrared spectra were data recorded the substituted metal (II) octa-methoxyphenylimino phthalocyanines a complex was recorded in KBr pellets and the reported results were presented in Table 2. In the range at 3424-3451cm-1 a broad absorption band were observed for all complexes and assigned to the H- bonding formed between nitrogen atom of the phthalocyanine molecules, hydrogen atom of moisture absorbed on KBr pellets during pelletization. The peak observed in the range 2362-2364cm-1 is due to C-H stretching vibration on the periphery of the phthalocyanine moiety. The sharp peak at 1628-1634cm-1 is assigned to C=N of imine group and the peaks in the 1380-1387cm-1 is due to C-N aromatic stretching frequency. The bands at 1045-1119, and 741-772cm-1 may be observed to the various phthalocyanines skeletal vibration.
Magnetic Susceptibility
The magnetic susceptibility and magnetic moments values of square planner M-Me PhIm PcO’s were investigated in the solid state over a range of 2.20-4.45 K Gauss and summary of the results were in Table-1. The values reported are the average of three independent determinations. The magnetic susceptibility were studies revealed that Cu Me PhIm PcO and Co Me PhIm PcO are paramagnetic and Ni Me PhIm PcO and Zn Me PhIm PcO are diamagnetic complexes.
Table 1: Elemental analysis and magnetic susceptibility data of metal (II) -octa- methoxyphenylimino phthalocyanines
| Complex (Yield) Colour | Empirical formulae.(Formula weight) | Field strength K Gauss | Magnetic susceptibility (cm χm10-6 cgs units) | Magnetic momentsμeff (B.M) | Elemental analysis (%)found (calcd) |
| CoMePhImPcO(86%)Dark green | C96H72N16O8Co(1634.93) | 2.202.663.103.584.01 | +2642.28+2439.08+2103.52+1937.02+1804.08 | 2.852.752.642.512.40 | C, 70.28;(70.46)H, 4.35; (4.40)N, 13.65; (13.70)Co, 3.56; (3.60) |
| CuMePhImPcO(85%)Dark green | C96H72N16O8Cu(1639.54 ) | 2.202.663.103.584.01 | +2610.10+2471.16+2125.54+1939.46+1825.62 | 2.782.652.572.472.42 | C,70.13;(70.26)H, 4.24;(4.39)N, 13.51;(13.60)Co, 3.72;(3.87) |
| NiMePhImPcO(79%)Dark Green | C96H72N16O8Ni(1634.69) | 2.66 | -766.81 | — | C, 70.25; (70.47)H, 4.23; (4.40)N, 13.62; (13.70)Co, 3.49; (3.59) |
| ZnMePhImPcO(74%)Green with brown ting | C96H72N16O8Zn(1641.38) | 2.66 | -849.00 | — | C, 70.04; (70.18)H, 4.13; (4.38)N, 13.42; (13.6)Zn, 4.72; (4.98) |
The observed magnetic moments for Cu Me PhIm PcO and Co Me PhIm PcO are higher than the expected only spin value corresponding to one unpaired electron (1.73 BM), mixing of higher energy degenerate states with ground state orbital and intermolecular co-operative effects. The higher magnetic moment then spin only value is due to the orbital contribution to µeff which may arise as a result of mixing of ground state orbitals (b2g)2, (eg)4, and (a1g)1 with higher orbitally degenerate states (b2g)2, (eg)3 and (a1g)2. The orbital contributes is consists of higher at magnetic field then that of higher one evidenced by higher µeff values at lower field strength attributed to intermolecular magnetic interaction coupled with magnetic anisotropy of phthalocynanines p electronic current as reported in the literature. The crystallographic studies that the metal phthalocyanines of Cu, Co, Ni and Zn has square planar structure with D4h symmetry and are isomerphous.
The molecular plan is approximate normal to a-b plane and molecules were stacked along the short b-axis. The molecular planes are inclined to the a-c axis at an angle of 45o . Thus the complexes stacked in column with N-atoms above and below on every metal atom (Fig.2) Hence the nearest neighbouring molecule along the b-axis contributes N-atom at the interplanar distance 3.8 Ao .
The overlapping of two neighboring molecules depends on the crystal modification. The XRD data reveals that the M-Me PhIm PcO’s as in β-form. The substituted metal (II) of one of the phthalocyanine overlaps with N-atom of azamethine atom of the other metal phthalocyanine molecule.
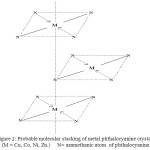 |
Figure 2: Probable molecular stacking of metal phthalocyanine crystal, (M = Cu, Co, Ni, Zn.) N= azamethanic atom of phthalocyanine. Click here to View figure |
Powder XRD
The spectra of M-Me PhIm PcO’s in the range of 2q angles 6-70o showed are not identical peaks Table -2. It is observed two peaks. One of the sharp peak at lower angle with maximum intensity and the other peak at higher angle with higher intensity. The inter- planar spacings on these angle gave the following values. Co-Me PhIm PcO 3.49, 27.97 Å; Cu-Me PhIm PcO 2.82, 45.52 Å; Ni-Me PhIm PcO 3.74, 27.93 Å and Zn-Me PhIm PcO 3.57, 27.94 Å, clearly indicating the crystanilline nature of the complex. The observed patterns are very much similar to those of the unsubstituted parent phthalocyanines except the broadening of the peaks in the case of M-Me PhIm PcO’s which may be due to the presence of substituents and which seems to play an significant role in the stacking of the substituted metal (II) phthalocyanine derivatives. X-ray diffraction patterns are used only to explain crystallinity qualitatively.
Table 2: Electronic, IR and powder XRD data of metal (II) octa methoxyphenylimino phthalocyanines.
| Complex | UV-visible absorption λ nm (logÎ) | IR-Spectral Data (cm-1) | Powder. XRD data 2q angle (d Å) | Relativeintensity (%) |
| CoMePhImPcO | 218 (4.82)260 (4.80)335 (5.42)593 (5.07)749 (5.20) | 741,1045,1170,1382,1634,1711,
2362, 3451. |
27.77, (3.49)28.93, (3.11)24.53, (3.40)31.30, (2.72)
|
100.0079.3649.2531.46
|
| CuMePhImPcO | 214 (4.92).265 (4.79)337 (5.43)584 (5.02)747 (5.14) | 772,1090,1387,1634,1718,2362,
3442. |
45.52, (2.82)31.72, (2.84)43.77, (2.73)25.96, (3.45) | 100.0080.2852.4434.27 |
| NiMePhImPcO | 226 (4.90)257 (4.63)328 (5.06)582 (4.44)726 (4.61) | 759,1119,1363,1401,1628,2223,
2362, 3424. |
27.93, (3.74)28.82, (3.11)23.25, (3.45)42.76, (2.83) | 100.0079.2444.3229.54 |
| ZnMePhImPcO | 219 (5.58)251 (5.46)327 (5.41)577 (5.39)728 (5.04) | 769,1121,1380,1632,2325,2364,
3441. |
27.94, (3.57)28.88, (3.03)42.96, (2.75)20.52, (4.37) | 100.0080.8150.2435.91 |
Thermogravimatric and Kinetic Studies
Thermogravimatric analytical data of octa-methoxyphenylimino phthalocyanine complexes both in air and nitrogen atmosphere are summarized in the Table 3 and 4. It is observed that the decomposition of the above complexes occurs generally in two steps, revealed that the initial weight loss of 2-3% corresponding to moisture. The first step degradation in air, which takes place in the temperature range of 250-350oC, may be accounted for the loss of four substituted imine groups. The major weight loss is observed for all the complexes in the second step in the temperature ranges of 350-600oC corresponds to the oxidative degradation of remaining four substituted imine groups and phthalocyanine moiety. Analysis of the final charred residue corresponds to the corresponding metal oxides. The thermal decomposition of imino substituted metal phthalocyanine complexes in the nitrogen atmosphere appears to be very slow. For Co-Me PhIm PcO, 69% of the complex was found to be decomposed at 700oC. For Cu-Me PhIm PcO, Ni-Me PhIm PcO about 55%, 54% and 43% loss of the mass was observed for Zn-Me PhIm PcO. Above trend conforms the relatively stability of these complexes in the order Co-Me PhIm PcO >Cu-Me PhIm PcO > Ni-Me PhIm PcO > Zn-Me PhIm PcO. DTA results revealed that all degradation steps are exothermic in nature. Kinetic and thermodynamic parameters of the title complexes have been evaluated by Boride’s method.
Table 3: TGA data of Cu(II), Co(II), Ni(II) and Zn(II) -octa-methoxyphenylimino phthalocyanines.
|
Complex |
Temperature of |
Mass Loss |
Probable mode of |
|
|
Decomposition oC |
(%) Found |
(%)Calcd |
Fragmentation |
|
|
CuMePhImPcO |
250-350 |
29.83 |
30.05 |
8-Imino groups |
|
350-600 |
65.45 |
65.84 |
Pc moiety |
|
|
CoMePhImPcO |
250-350 |
29.81 |
30.17 |
8-Imino groups |
|
350-600 |
65.94 |
65.04 |
Pc moiety |
|
|
NiMePhImPcO |
250-350 |
29.77 |
30.16 |
8-Imino groups |
|
350-600 |
65.86 |
65.18 |
Pc moiety |
|
|
ZnMePhImPcO |
250-350 |
29.65 |
30.14 |
8-Imino groups |
|
350-600 |
65.53 |
65.14 |
Pc moiety |
|
Plot of ln(ln1/y) versus 1/T (where y is the fraction of the complex not decomposed) were developed for the decomposition segment where loss of functional groups occur. From the plots, the energy og activation(Ea) and frequency factor (lnA) were evaluated. Enthalpy (∆H), Entropy(∆S) and free energy (∆G) have been computed using standard equation in Table 4.
Table 4: Kinetic and thermodynamic parameters of octa-methoxyphenylimino phthalocyanines of Cu, Co, Ni and Zn in air and inert atmosphere.
| Compounds | Activation energy E0KJ/mole | Frequency factor. lnA min-1 | ∆H KJ/mole | ∆SJ/K | ∆G KJ/mole |
| CuMePhImPcOIII |
5.86 (0.74) 3.73 (1.52) |
6.16 (2.02) 5.37 (5.46) |
2.72 (-1.28) -0.74 (-2.42) |
-165.04 (-160.03) -152.26 (-149.74) |
62.41 (61.53) 81.66 (79.54)
|
| CuMePhImPcOIII |
0.88 (0.67) 4.66 (1.25) |
6.36 (3.38) 7.48 (4.65) |
-1.37 (-1.11) 2.87 (-1.32) |
-176.06 (-174.11) -143.93 (-141.97) |
74.94 (74.78) 79.53 (77.26)
|
| NiMePhImPcOIII |
1.44 (0.71) 5.85 (1.53) |
5.22 (2.25) 4.44 (4.83) |
-1.24 (-0.78) -0.86 (-0.84) |
-159.63 (-157.58) -149.76 (-143.64) |
72.87 (70.56) 81.74 (80.23)
|
| NiMePhImPcOIII |
1.94 (0.67) 8.55 (5.53) |
3.49 (2.38) 7.97 (6.45) |
-0.96 (-1.48) 2.86 (-1.52) |
-175.96 (-173.35) -142.74 (-140.43) |
75.26 (74.96) 82.45 (80.92) |
(I and II corresponds to the I and II stage of degradation and the values in the parenthesis are for nitrogen atmosphere)
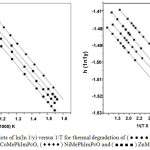 |
Figure 3: Plots of ln(ln 1/y) versus 1/T for thermal degradation of (● ● ● ●) Cu Me PhIm PcO, (● ● ● ●) Co Me PhIm PcO, ( ♦ ♦ ♦ ♦ ) Ni Me PhIm PcO and (■ ■ ■ ■) Zn Me PhIm PcO in air. Click here to View figure |
Conclusion
A simple and convenient method has been optimized for the synthesis of pigments -octamethoxy phenylimino phthalocyanines of Cu (II), Co(II), Ni(II) and Zn(II). Magnetic susceptibility studies revealed the paramagnetic behavior of Cu(II) and Co(II) octa methoxy phenylimino phthalocyanine derivatives and the variation of magnetic moments with magnetic field indicated the presence of intermolecular co-operative effect. Thermogravimatric analysis of the complexes in an inert atmosphere revealed the stability in the order Cu Me PhIm PcO > Co Me PhIm PcO > Ni Me PhImPcO > Zn Me PhIm PcO.
References
- Achar, B.N.; J.M. Bhandari, J.M. Synth. React. Inorg. Metal.-Org. Chem.1993, 23(1),133- 148
CrossRef - Achar, B.N.; G.M. Fohlen,; Parker, J.A,; Keshavayya, J. Polyhedron.1987, 6 (6),1463-1467
CrossRef - Broido, A. J. Polym Sci, 1969, Part A-2 (7), 1761-1773
- Gregory, P. J. Porphyrins and Phthalocyanines. 2000, 3(4), 432
CrossRef - Hanack, M,; Lang, M. Adv. Mater. 1994, 6, 819-833
CrossRef - Leznoff, C.C,; Lever, A. B. P. Phthalocyanines, Properties and Applications, . 1–4, VCH, New York.1996
- Moinuddin Khan, M.H,; Fasiulla,; Harish, M. N.K,; Keshavayya, J,; Venugopla Reddy, K.R.J. Coordination. Chem.2007, 60(12), 1225-1276
- Selwood, P. Magneto chemistry, Interscience, New York.1956
- Somashekarappa, M.P,; Keshavayya, J. Synth React Inorg Met-Org.Chem.1999, 29(5),767-783
CrossRef - Venkataraman, K. The Chemistry of Synthetic Dyes, Academic Press, New York.1952, 2
- Venugopala Reddy, K.R,; J. Keshavayya, J. Synth React Inorg Met-Org Chem.2002, 32 (7), 1235-1244
CrossRef - Venugopala Reddy, K.R,; Keshavayya, J. Turk. J.Chem.2002, 26(4), 573-580
- Venugopla Reddy, K.R,; M.N.K. Harish, M. N. K,; Fasiulla, Moinuddin Khan, M/H,; Keshavayya, J. J. Fluorine Chem.2007, 128, 1019-1025.
- Wright, J.D. Prog Surf. Sci, 1989, 31(1)
- Someshakarappa, M.P,; Keshavayya. J. Spectrochemica Act. 2003, 59- 67
- Venugopala Reddy, K.R,; Keshavayya, J. Dyes and Pigments.2002, 187-194
CrossRef - Moinuddin Khan, M.H,; Harish, Keshavayya, J,; Venugopla Reddy, K.R.J. Coordination. Chem.2009, 62(5), 854-862

This work is licensed under a Creative Commons Attribution 4.0 International License.









