Silver Bullet for the Computation of Equivalent Weight of Sodium Thiosulphate in the Reaction 2S2O2-3 + I2 → S4O2-6 +2I- A Chemical Education Article for Freshmen Student
Sanjeev R1, V. Jagannadham2, Veda Vrath R3 and V. E. M. Mamatha Bethapudi1
1Department of Chemistry, Geethanjali College of Engineering and Technology, Cheeryal, Medchal District, 501301, Telangana, India.
2Department of Chemistry, Osmania University, Hyderabad-500007, India.
3Department of Chemistry, L N Gupta Evening College, Hyderabad-500002, India.
Corresponding Author E-mail: rachuru1sanjeev1@rediffmail.com
DOI : http://dx.doi.org/10.13005/ojc/330566
When we standardize sodium thiosulphate solution either by iodometry or by a iodimetry, we base our understanding on![]() While addressing the freshmen students, especially during the pre-experimental lectures, we teach them the computation of equivalent weight of sodium thiosulfate (hypo); this necessitates the knowledge of the difference in oxidation state of sulphur atoms in the product (2.5) and the reactant side (2.0); the difference in oxidation state of sulphur atoms is 0.5. The overtly observable query which occurs to the students is, “Is the equivalent weight and molecular weight of sodium thiosulphate same or different?” If yes, then the change in the oxidation state apparently does not conform to the difference, 0.5. This article deals with this apparently simple but extremely perplexing question.
While addressing the freshmen students, especially during the pre-experimental lectures, we teach them the computation of equivalent weight of sodium thiosulfate (hypo); this necessitates the knowledge of the difference in oxidation state of sulphur atoms in the product (2.5) and the reactant side (2.0); the difference in oxidation state of sulphur atoms is 0.5. The overtly observable query which occurs to the students is, “Is the equivalent weight and molecular weight of sodium thiosulphate same or different?” If yes, then the change in the oxidation state apparently does not conform to the difference, 0.5. This article deals with this apparently simple but extremely perplexing question.
Standardize; Pre-experimental; Thiosulfate; Apparently
Download this article as:| Copy the following to cite this article: Sanjeev R, Jagannadham V, Vrath V. R, Bethapudi V. E. M. M. Silver Bullet for the Computation of Equivalent Weight of Sodium Thiosulphate in the Reaction 2S2 O2,3- + I2 → S4 O2,6- + 2I- A Chemical Education Article for Freshmen Students. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Sanjeev R, Jagannadham V, Vrath V. R, Bethapudi V. E. M. M. Silver Bullet for the Computation of Equivalent Weight of Sodium Thiosulphate in the Reaction 2S2 O2,3- + I2 → S4 O2,6- + 2I- A Chemical Education Article for Freshmen Students. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=37451 |
Introduction
If one attempts to calculate the equivalent weight of hypo in the title reaction i.e. the reaction between sodium thiosulphate and iodine molecule, the conventional method involves the evaluation of the oxidation state of sulphur in the reactant and product sides; this in essence gives the change in the number of electron(s) in the reaction. Then finally, we divide the molecular weight of sodium thiosulphate by the change in the number of electrons.
Discussion
Let us apply the conventional method for the computation of equivalent weight of sodium thiosulphate to the title reaction. The first step involves the evaluation of oxidation states by the traditional method [1]. The oxidation state of sulphur (x) in
![]()
is {2x + 3(-2) = -2, hence x = 2} 2. The oxidation state of sulphur (x) in
![]()
is {4x + 6(-2) = -2, hence x = 2.5} 2.5. An important fact one should not forget is that, the oxidation states which we have evaluated are average oxidation states of the sulphur atoms. The difference in oxidation state is 0.5. Further, the next step is the division of molecular weight of sodium thiosulphate by 0.5; if the hypo is associated with 5 molecules of water, then the molecular weight of sodium thiosulphate is 248 and thereby the equivalent weight would be 248/0.5 = 496 which is absurd and erroneous. Thus, the conventional method for the evaluation of equivalent weight in the title reaction fails miserably. In fact this is how the students carry out the evaluation of equivalent weight of hypo and get erroneous results. A very important point one should bear in mind when applying the traditional method of determination of oxidation states is that, the practice involves using of set of certain rules, the choice of which is arbitrary. Hence the method might not be applicable to each and every molecule; the assigning of oxidation states would fail particularly in complex molecules [2]. Further it goes without saying; the traditional method fails for our title reaction. Now, the daunting question is how do we compute the oxidation states of the reactant thiosulfate and the product tetrathionate, and finally equivalent weight of hypo for the title reaction?
To answer the question in the foregoing paragraph let us draw the structures of the reactant and the product of the title reaction as shown in scheme 1:
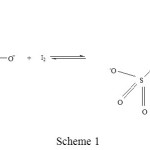 |
Scheme 1 |
One of the rules for the computation of oxidation states is described as: For covalently bound atoms, the oxidation state is obtained by assigning the shared electrons to the more electronegative atom and then counting the charge on the quasi-ion. Electron pairs shared by atoms of the same element are divided equally. Based on this rule, for the reactant thiosulfate ion, the oxidation states of the outer and the inner sulphur atoms are assigned -1 and +5 respectively. These oxidation states of -1 and +5 are reinforced by the results obtained by XANES spectroscopic method [3]. And for the product tetrathionate molecule the terminal sulphur atoms, which are actually the inner sulphur atoms of the thiosulfate (the sulphur atoms to which two oxygen atoms and one O– are bonded), have the oxidation states of +5 each; for the central sulphur atoms which are actually the outer sulphur atoms of the thiosulfate (i.e. the sulphur atoms to which only sulphur atoms are bonded), the oxidation states are 0 for each of the two sulphur atoms. If one carefully observes the inner sulphur atoms of the reactants, as they proceed to form the tetrathionate molecule, the oxidation state is intact, i.e. +5 oxidation states are unchanged. Further if one carefully observes the outer sulphur atoms of the reactants, as they proceed from reactant to product the oxidation states of the two sulphur atoms changes from -1 to 0. The change in oxidation state of each of the sulphur atom is 0 – (-1) = +1. In essence there is no change in the oxidation state of inner sulphur atoms of thiosulfate ion, and the outer sulphur atoms of each of the thiosulfate undergoes a change of +1. Hence we are aptly justified in taking the average of change in oxidation state of the two outer sulphur atoms which is {+1 + (+1)}/2 = +1. Finally the equivalent weight of thiosulfate ion (normally we take sodium thiosulfate which is associated with 5 water molecules, and thus the molecular weight is 248) or hypo salt is 248/(+1) = 248. Hence the equivalent weight and the molecular weight of hypo in this reaction is the same.
Conclusion
If we apply the traditional method for the computation of oxidation states, we get erroneous and an absurd result. But when we employ the method described in the foregoing paragraph, we are aptly able to justify the equivalent weight and the molecular weight of sodium thiosulphate salt. Thus the perplexing drawback of the traditional method is surmounted.
References
- Raymond Chang. Chemistry; Ninth Indian Ed.; Tata McGraw Hill, 2008, 135.
- Vairavmurthy, A. Using X-ray absorbtion to probe sulfur oxidation states in the complex molecules. Spectrochimica Acta Part A. Elsevier. 1998, 54, 2009-2017.
- Vairavmurthy, A.; Manowitz, B.; Luther III, G. W.; Jeon, Y . Oxidation state of sulfur in thiosulfate and implications for anaerobic energy metabolism. Geochimica Cosmochimica Acta 1993, 57, 1619-1623.

This work is licensed under a Creative Commons Attribution 4.0 International License.









