Mild Acid Ultrasonic Assisted Extraction of Arsenic Residues in Different Parts of Hot Chilli Prior to Ultra-Trace Determination by flow Injection-Hydride Generation Atomic Absorption Spectrometry
Sugunya Phongsirirux, Phitchan Sricharoen, Nunticha Limchoowong and Saksit Chanthai
Materials Chemistry Research Center, Department of Chemistry and Center of Excellence for Innovation in Chemistry, Faculty of Science, Khon Kaen University, Khon Kaen 40002, Thailand.
Corresponding Author E-mail: sakcha2@kku.ac.th
DOI : http://dx.doi.org/10.13005/ojc/330526
Mild acid ultrasonic assisted extraction (UAE) of arsenic residues in chilli was introduced as a green approach due to simple, efficient and rapid method in association with flow injection-hydride generation atomic absorption spectrometry (FI-HGAAS). Arsenic species, AsIII and AsV, were determined in different parts of chilli fruits including seed, pericarb and placenta. This procedure leads to improve mild the extraction conditions using 0.1 g of ground sample in 10 mL of 1 M acid mixture of HCl : HNO3 (3:1, v/v) and 1.5 mL H2O2 for 15 min sonication. Analytical features of merit for both FI-HGAAS and flame atomic absorption spectrometry (FAAS) measurements were obtained and compared. Actually, trace amounts of total As in these samples were found very lower than limit of quantitation of FAAS. Therefore, the ultra-trace amounts of AsIII, AsV and total As in the chilli samples determined by FI-HGAAS were found in the ranges of 1.9-10.2 ng g-1, 1.2-19.1 ng g-1, 3.3-22.4 ng g-1, respectively. Among three varieties of these chilli samples, higher contents of As were mostly found in seed of the fruits. The proposed method is, therefore, a novel one and can be implied for a routine work for arsenic in fruits and vegetables with its emerging applications.
KEYWORDS:Ultrasonic Assisted Extraction; Hydride Generation Atomic Absorption Spectrometry; Chilli; Arsenic
Download this article as:| Copy the following to cite this article: Phongsirirux S, Sricharoen P, Limchoowong N, Chanthai S. Mild Acid Ultrasonic Assisted Extraction of Arsenic Residues in Different Parts of Hot Chilli Prior to Ultra-Trace Determination by flow Injection-Hydride Generation Atomic Absorption Spectrometry. Orient J Chem 2017;33(5). |
| Copy the following to cite this URL: Phongsirirux S, Sricharoen P, Limchoowong N, Chanthai S. Mild Acid Ultrasonic Assisted Extraction of Arsenic Residues in Different Parts of Hot Chilli Prior to Ultra-Trace Determination by flow Injection-Hydride Generation Atomic Absorption Spectrometry. Orient J Chem 2017;33(5). Available from: http://www.orientjchem.org/?p=38790 |
Introduction
Chilli (Capsicum annuum L.) is an important fruit industrial crop of Thailand’s economy because of the combination of color, flavor, taste and nutritional value1. A large number of biological properties and potential fitness benefits of consuming chilli have been examined such as free radical scavengers, anti-inflammatory, anti-neoplastic, anti-arthritic, anticancer and antifungal properties2. At a global level, environmental pollution due to heavy metals has become a major concern. Arsenic (As) is natural occurring chemical compound. It is also a poisonous metal that can be present inorganic and organic forms at various levels in water, soil, dust, wood, plant and other material. Exposure to As is associated with skin lesions and increased risk of developing cancer of the skin, lungs, liver, kidney and bladder3. Arsenic can also occur as residue in chilli due to it can be grown in any variety of soils and people can be exposed to this metal from ingesting contaminated food or water. It accumulation in the body can lead to harmful effects over time, depending on the dose and toxicity4,5. Thus, it is important to determine the species of arsenic in food because of their high toxicity. Various instrumental techniques, such as flame atomic absorption spectrometry (FAAS) 6, inductively coupled plasma-optical emission spectrometry (ICP-OES) 7,8, inductively coupled plasma-mass spectrometry (ICP-MS) 9, Flow injection-hydride generation atomic absorption spectrometric (FI-HGAAS) 10, UV-Vis spectrophotometry11, voltammetric method12 and colorimetric incorporated with image processing13 have been used to accurately and precisely determine As at trace levels in various samples.
Ultrasound-assisted extraction (UAE) is widely used in analytical chemistry to facilitate various steps some analytical process, particularly sample preparation. They are expeditious, low-cost, and efficient alternatives to old-fashioned extraction techniques14. UAE may improve extraction efficiency through disruption of cell walls, particle-size reduction, and improved mass transfer of the cell contents as a result of cavitation. UAE is an easy, convenient, and rapid way of desorbing inorganic and organic contaminants from sediments, soils and natural samples15,16.
This study was aimed to investigate the optimum conditions of UAE under mild acid solution from three varieties of hot chilli as a whole with respect to their seed, pericarb and placenta for the determination of arsenic(III) and arsenic(V) by FI-HGAAS and Total arsenic by FAAS.
Materials and Methods
Chemicals and Reagents
AsIII stock solution (1000 mg L−1), AsV stock solution (1000 mg L−1), and hydrogen peroxide (30%, w/w) were obtained from Ajax fine chem (Australia). Hydrochloric acid (37%, w/v) and sodium hydroxide were from Carlo Erba (France). Sodium borohydride was from Lab Chem (France). Nitric acid (65%, w/v) was from Lab Scan Asia (Thailand). Thiourea was from BDH laboratory supplies (England). A Millipore water purification system (Molsheim, France) was used to obtain deionized water with a resistivity of 18.2 MΩ cm.
All glassware was cleaned by soaking in dilute 1% (v/v) HNO3 overnight and rinsed two times with deionized water prior to use.
Instruments
Arsenic measurements were made using an atomic absorption spectrometer of the Perkin-Elmer Instrument AAnalyst 100 (Connecticut, USA) equipped with a flow injection analysis system Model FIAS-100 (Perkin Elmer Instruments, USA), used for continuous flow hydride generation. The FIAS-100 flow injection system consists of one peristaltic pump, five-port valve and a regulated gas control. Argon gas was used as carrier gas for the transposition of arsine (AsH3) from the gas-liquid separator to the electrically heated quartz tube. The electrodeless discharge lamp was used for this determination. The atomic absorption signal was measured as a peak height mode providing an analytical curve. The peristaltic pump, injection time and data acquisition were controlled through Perkin Elmer AAwinlab atomic absorption software version 3.2.
Ultrasound assisted extraction was done in an ultrasound bath at a maximum power of 640 W and a frequency of 35 kHz (Sonorex Digitec DT 510 H, Bandelin, Germany).
Sample Materials
Chilli samples were obtained from Department of Plant Science and Agricultural Resources, Faculty of Agriculture, Khon Kaen University, Khon Kaen, Thailand. Three varieties of them were used in this study including the so-called “Yodson Mordindaeng”, “Super-hot” and “Erawa”. The samples were soaked and washed clean with water. They were dried in an oven at 60°C for 48 h. Each kind of the sample was grouped sampling as a whole and the fruit was also separated in different parts as seed, pericarb and placenta. The ground samples in sealed plastic bag were stored in desiccator before use.
Sample Preparation
The sample preparation was prepared by an ultrasonic assisted extraction (UAE) method for the arsenic speciation determination by FI-HGAAS. Sample amounts of 0.2 g were weighed and transferred into a 50 mL polypropylene bottles followed by 10 mL of the mixed acid leaching solution (7.5 mL of 1M HCl and 2.5 mL of 1 M HNO3). After that, 1.5 mL of H2O2 (30%, v/v) was added. The extraction bottles were then subjected individually to an ultrasound energy corresponding to ultrasonication for 15 min to leach the arsenic from the sample into the acid solution. After sonication, the obtained acid extracts were separated from the remaining solid materials using centrifugation at 5000 rpm for 5 min, followed by filtration using Whatman filter paper No. 1 (Ø 55 mm) into 10 mL volumetric flask. The optimum conditions for this sample preparation were certainly studied by the UAE method. The parameters affecting on the absorbance of As were investigated as follows.
Effects of Sample Amount and Solvent Volume
The effect of sample amount was investigated by varying the amounts of the ground chilli sample ranging from 0.05-0.40 g. The solvent volume (of the dilute acid) was varied with 5, 10, 15 and 20 mL according to the fixed ratio of the sample amount.
Effects of Type of Dilute Acid and Acid Concentration
Various types of the dilute acid solution were investigated for an extraction solvent including hydrochloric acid, nitric acid and their mixed ratios of these acids. The acid concentration of each of HCl and HNO3 or the mixed ratios of these acids was studied in the range of 1-6 M.
Effect of Hydrogen Peroxide
In addition, volume of 30% (w/w) hydrogen peroxide (H2O2) used as auxiliary mild oxidation was investigated in the range of 0-10 mL.
Effect of Extraction Time
The effect of extraction time was investigated by varying the ultrasonic sonication time from 1-20 min.
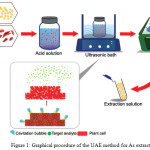 |
Figure 1a: Graphical procedure of the UAE method for As extraction. Click here to View figure |
Arsenic Speciation Analysis in Real Sample
A 5 mL of the sample extract was pipetted into 10 mL volumetric flask and made up the final volume with 10% (v/v) hydrochloric acid prior to determination by FI-HGAAS by using its calibration graph of AsIII standard solution. A 5 mL of the sample extract was pipetted into 10 mL volumetric flask. After that, 1 mL of 2% (w/v) thiourea solution was added and diluted to the mark with 10% (v/v) hydrochloric acid. This extract solution was left to stand with common hand shake for 30 min at room temperature prior to determination by FI-HGAAS by using its calibration graph of AsIII standard solution in the presence of 2% (w/v) thiourea solution. Since AsV will be reduced to be AsIII for AsH3 determination, the content of AsV is then calculated by difference in total inorganic As and that of original AsIII. Alternatively, total As content in the sample extracts can be determined directly by FAAS for comparison, if their trace amounts are found in the instrument’s linear range. The accuracy of the proposed method was expressed in terms of recovery. The recovery was studied by spiking a known concentration of standard solution into samples before analysis in three replicates. The recovery study of each arsenic species was investigated. The AsIII and AsV at the concentration of 10 µg L-1 were spiked into the real samples.
Determination of Arsenic(III), Arsenic(V) and total Arsenic by FI-HGAAS and FAAS
At least five step dilutions for working solution of 10 µgL-1 (ppb) AsIII was prepared from its stock solution (1000 mgL-1) in 25 mL volumetric flask with with 10% (v/v) hydrochloric acid. For 10 µgL-1 (ppb) working solution of AsV, it was also prepared in the same manner as done for AsIII. The working solution of AsIII or AsV was prepared daily. The calibration standard curve was established using the standard solution prepared in 10% (v/v) hydrochloric acid by dilution from the working solution of AsIII. The standard AsIII solution was obtained with the series of 1.0, 2.0, 3.0, 4.0 and 5.0 µgL-1 prior to determination by FI-HGAAS.
For AsV analysis, the calibration standard solution was prepared with various volumes of the AsV working solution, which was pipetted into 10 mL volumetric flask. After that, 1 mL of 2% (w/v) thiourea solution was added and then diluted to 10 mL with 10% (v/v) hydrochloric acid. Various concentrations of the AsV solution were also prepared daily in the series of 1.0, 2.0, 3.0, 4.0 and 5.0 µgL-1. The AsV content was then calculated from difference in their contents between total As and AsIII.
The pre-reducing agent to reduce AsV to AsIII species was adopted from previous report (Thaharn et al., 2014). A 2.0% (w/v) thiourea solution was prepared by dissolving 2.0 g of thiourea (MW 76.12 g mol-1) to 100 mL volumetric flask with deionized water. The thiourea solution was prepared daily. A 5.00 mL of the AsV working solution was pipetted into 10 mL volumetric flask. After that, 1 mL of 2% (w/v) thiourea solution was added and then diluted to 10 mL with deionized water. This solution was left to stand for 10-60 min at room temperature prior to determine by FI-HGAAS.
A 0.5% (w/v) sodium borohydride was prepared by dissolving an accurate amount of NaBH4 in 0.04% (w/v) NaOH. This solution was freshly prepared prior to use each time. A 1% (v/v) hydrochloric acid solution was prepared by dilution of 27.02 mL of 37% (w/v) hydrochloric acid to 1000 mL volumetric flask with deionized water. A 10% (v/v) hydrochloric acid solution was prepared by dilution of 270.26 mL of 37% (w/v) hydrochloric acid to 1000 mL volumetric flask with deionized water.
Alternatively, total As could be determined directly by flame atomic absorption spectrometry (FAAS), if its content laid in the linear calibration curve provided. The calibration standard was established using the standard solution of AsIII or AsV containing a series of 2.0, 4.0, 6.0, 8.0, and 10.0 mg L-1 prepared in 10% (v/v) hydrochloric acid.
The analytical characteristics of the proposed method for arsenic analysis were investigated under the optimum conditions. The studied parameters were linearity, limit of detection (LOD), limit of quantitation (LOQ), precision and accuracy.
Linearity was obtained from calibration plot. The calibration plots were studied by analysis of standard solution with various concentrations ranging from 0.5 to 100.0 µg L-1 of AsIII. LOD and LOQ of the proposed method were deduced based on the signal to noise ratio (S/N) of 3 and 10, respectively.
The precision of the present method was evaluated in terms of reproducibility and repeatability of the calibration slope. The repeatability (intra-day precision) was achieved by replicating measurements of the calibration slop in a day (n = 10). Whereas, reproducibility (inter-day precision) was calculated from the experiments carried out in ten consecutive days (n = 10).
Results and Discussion
Optimization of pre-reduction agent for Arsenic(III), Arsenic(V) and total Arsenic Determination
For inorganic arsenic speciation, AsIII in the sample extract must be determine selectively under the optimum conditions by FI-HGAAS. Then, using of a suitable pre-reducing agent to convert a residual part of AsV in the same sample to AsIII species, including an original AsIII, was conducted and analyzed as total As content. The AsV content was determined by difference in between total As and AsIII. At this point of view, the pre-reduction reaction of AsV in the sample extract must be completely done in association with constant sensitivity of their calibration curves for either AsIII in the absence or in the presence of the pre-reducing agent used. In addition, if necessary, choice of AsV in the presence of the pre-reducing agent was introduced, its sensitivity might be the same as those of AsIII as mentioned above. The reduction reaction time of 2% (w/v) thiourea was investigated in the range of 10-60 min as shown in Fig. 1. It was found that 30-40 min gave the highest absorbance among other in this experiment. Thus, 35 min was selected for further experiments.
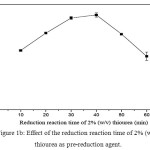 |
Figure 1b: Effect of the reduction reaction time of 2% (w/v) thiourea as pre-reduction agent. Click here to View table |
Optimization of Sample Preparation
The sample preparation for chilli by using UAE was carried out. The optimum conditions for UAE were also investigated in detail in order to reduce the plant matrices which might affect the extraction efficiency by varying sample amount, extraction time, type of dilute acid, effect of hydrogen peroxide, dilute acid concentration and volume of the extraction solution by a simple optimization method comparing its response in terms of absorbance (AU) of the As content in the selected chili samples.
Effect of Amount of Sample
The amount of sample was varied in the range of 0.05 to 0.4 g. The results are shown in Fig. 2(a). It was found that the absorbance of sample was higher than 0.0078 for seed of chilli. Thus, the amount of sample within this range could be used in this procedure. However, in this case, 0.1 g of the sample was chosen, because it could be applied for determination of arsenic in different parts of the dried chilli samples by both FI-HGAAS and FAAS methods.
Effect of Extraction Time
The extraction time is an important factor for sample extraction. This parameter was used for completion of extraction reaction of sample solution. In order to investigate the optimal extraction conditions, the extraction time was varied with 1, 5, 10, 15 and 20 min as shown in Fig. 2(b). From this result, the crude extraction was carried out using 15 min for the samples.
Effect of Type of Dilute Acid
Under the UAE procedure, the extraction of analytes from solid matrices can easily be performed using an acidic solution. The presence of hydrogen ion (H+) in liquid phase can leach out the metallic species from the solid sample into the liquid phase as inorganic ions17.Nitric acid is frequently reported in studies of the extraction of metals using ultrasonic energy. However, other acid solutions and their mixture have also been reported18. In this work, different acid solutions: HCl, HNO3 and two kinds of mixing ratio of HCl : HNO3 (1:1, 3:1, 1:3, by volume) each at 1.0 Mconcentration were chosen to investigate in details. The results obtained are shown in Fig. 2(c). It was found that high absorbance was obtained using 1 M HCl : HNO3 (3:1, v/v) solution compared with other acid solutions. Thus, next experiment was performed using 1 M HCl : HNO3 (3:1, v/v) solution as a mild acid extraction solvent.
Effect of Volume of the Extraction Solution
Under the UAE procedure, the volume of the extraction solution was also investigated ranging from 5, 10, 15 and 20 mL with the fixed sample amount of 0.2 g. The results obtained are shown in Fig. 2(d). It was evident that the absorbance value of AsIII was found higher than 0.0037 for the extraction of the seed sample. Therefore, 10 mL of the extraction solution was used as its appropriate ratio.
Effect of Hydrogen Peroxide
Hydrogen peroxide is a powerful oxidizing agent that acts via the formation of hydroxyl radicals, which preferably oxidize thiol groups in biomolecules. The volume of hydrogen peroxide was investigated ranging from 0, 0.5, and 1.0-10.0 mL with 1.0 mL interval. The absorbance for As determination increased slightly when increasing the volume of hydrogen peroxide from 1.0 to 1.5 mL as shown in Fig. 2(e). After that the volume reached 1.5 mL, giving a constant trend. Thus, 1.5 mL of hydrogen peroxide (30%, v/v) was preferably used for further experiment.
Effect of Dilute Acid Concentration
Different concentrations of the mixed ratio of HCl : HNO3 (3:1, v/v) (1, 2, 3, 4, 5 and 6 M) were used to evaluate their performance in the extraction procedure. The influence of these acid concentrations for HCl : HNO3 (3:1, v/v) solutions on the absorbance was evaluated by fixing the solution volume, sonication time, sample mass, ultrasonic power and bath temperature. The results obtained are shown in Fig. 2(f). It was found that 1 M HCl : HNO3 (3:1, v/v) solution gave relatively high absorbance. Thus, this acid mixture was preferably used for further experiment.
For UAE optimization conditions, it was found that the mild acid extraction could be done using 10 mL of the mixed ratio of 1 M HCl : HNO3 (3:1, v/v) and 1.5 mL hydrogen peroxide with at least 0.1 g sample for 15 min extraction time.
 |
Figure 2: Optimization of the extraction parameters. (a) Effect of the sample amount. (b) Effect of the extraction time. (c) Effect of different acid solution. (d) Effect of volume of the extraction solvent. (e) Effect of volume of hydrogen peroxide. (f) Effect of dilute acid concentration. Click here to View figure |
Analytical Performance Characteristics
The calibration curve and its regression equation were obtained for both AsIII and AsV standard solutions. The calibration curve was studied between 0.03 and 50.0 µg L-1 and the linearity was maintained from 1 to 5 µg L-1 with correlation coefficient (r2) greater than 0.9995. The LOD and LOQ were calculated as 3sb/S and 10sb/S respectively19; where sb is the blank standard deviation and S is the sensitivity of the method as obtained from the calibration slope. The LODs of FI-HGAAS method were 0.026 µg L-1 for AsIII and 0.037 µg L-1 for AsV. The LOQs of FI-HGAAS method were 0.086 µg L-1 for AsIII and 0.124 µg L-1 for AsV. For both LOD and LOQ of FAAS method, they were found to be 0.57 mg L-1 and 1.99 mg L-1, respectively. The precision of the present method is expressed in terms of relative standard deviation (RSD), estimated from 10 replicates and calculated at the concentration of 5 µg L-1 which was found to be 2.01% for AsIII and 3.92% for AsV.
Real Sample Analysis
The proposed method was applied for the trace determination of inorganic As species in different parts of dried chilli samples under the optimum conditions of UAE and FI-HGAAS. The contents of both AsIII and AsV including total As in three varieties of hot chilli are summarized in Table 1. The ultra-trace amounts of AsIII, AsV and total arsenic in these chilli samples were found in the ranges of 1.9-10.2 ng g-1, 1.2-19.1 ng g-1, 3.3-22.4 ng g-1, respectively. The recovery was studied by spiking of each 10 µg L-1 standard solution of AsIII, AsV and total As into different parts of dried chilli samples for FI-HGAAS measurement and also spiking of each 10 mg L-1 standard solution of AsIII into their different parts of these samples for such FAAS measurement. The recovery was calculated as follows20,21:
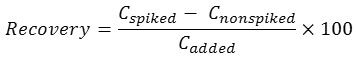
where Cspiked, Cnonspiked, and Cadded are the concentration of the analyte after addition of a known amount of standard in the real sample, concentration of the analyte in the real sample, and concentration of a known amount of standard that was spiked in the real sample, respectively.
Under the optimized conditions for the UAE, the method recoveries of total arsenic found in different parts of “Yodson Mordindaeng” hot chilli such as seed (93.82 ± 2.37%), pericarb (94.93 ± 0.06%), placenta (97.57 ± 3.25%) and Whole (101.6 ± 3.08%) determined by FAAS were obtained. Emphasizing on some varieties of the hot chilli, the recoveries of AsIII for three kinds of the chilli samples used were found in the range of 80.00 to 98.33%. Those of AsV were ranged of 79.33 to 97.67%, and total As were also found to be 79.50 to 98.83% as summarized in Table 2.
Table 1: Recovery of spiked 10 µg L-1 of AsIII, AsV and total As in different parts of chilli samples for AsIII, AsV and total As determination under the optimized conditions of UAE and FI-HGAAS methods
| Sample |
Recovery (%) ± RSD (%); n = 6 |
||
|
|
AsIII |
AsV |
Total As |
| Yodson Mordindaeng | |||
| Seed |
91.17±5.69 |
96.67±4.51 |
93.00±1.79 |
| Pericarb |
98.33±4.25 |
91.17±4.06 |
95.50±3.90 |
| Placenta |
97.83±4.89 |
92.67±3.38 |
94.83±4.59 |
| Whole |
81.83±5.37 |
90.33±3.61 |
82.33±5.13 |
| Super-hot | |||
| Seed |
80.67±8.54 |
79.33±7.20 |
88.33±9.10 |
| Pericarb |
82.50±5.56 |
87.33±2.13 |
79.50±7.23 |
| Placenta |
95.00±4.26 |
91.67±4.29 |
92.50±3.53 |
| Whole |
80.83±4.31 |
90.50±6.27 |
84.67±3.13 |
| Erawa | |||
| Seed |
90.17±9.75 |
87.00±9.50 |
94.83±8.62 |
| Pericarb |
80.00±6.56 |
89.00±1.00 |
89.00±2.84 |
| Placenta |
80.00±2.23 |
79.50±3.87 |
82.00±5.28 |
| Whole |
95.83±4.34 |
97.67±5.85 |
98.83±1.85 |
– : not detectable (< LOQ)
Table 2: The ultra-trace contents of arsenic species in different parts of chilli samples (mea n± SD) (n = 6)
| Sample |
Concentration (ng g-1) ± SD ; n = 6 |
||
|
|
AsIII |
AsV |
Total As |
| Yodson Mordindaeng | |||
| Seed |
2.6±0.2 |
17.9±0.1 |
20.8±0.8 |
| Pericarb |
– |
– |
– |
| Placenta |
– |
– |
– |
| Whole |
3.3±0.1 |
19.1±0.4 |
22.4±0.3 |
| Super-hot | |||
| Seed |
4.1±0.4 |
2.5±0.6 |
6.6±0.3 |
| Pericarb |
2.3±0.6 |
2.1±0.3 |
4.5±0.5 |
| Placenta |
– |
– |
– |
| Whole |
6.4±0.6 |
5.3±0.6 |
11.8±0.3 |
| Erawa | |||
| Seed |
6.3±0.3 |
6.5±1.9 |
12.7±0.2 |
| Pericarb |
1.9±0.1 |
4.2±0.2 |
6.2±0.2 |
| Placenta |
2.1±0.2 |
1.2±0.6 |
3.3±0.4 |
| Whole |
10.2±0.2 |
12.3±0.9 |
22.4±0.6 |
– : not detectable (< LOQ)
Conclusion
UAE as a green approach was described for simple, efficient and rapid determination at ultra-trace level of arsenic in different parts of hot chilli by FI-HGAAS. Their optimized conditions of UAE are certainly investigated in details, in particular several variables that could affect the performance of the extraction method. In this study, arsenic species, AsIII and AsV were determined in three varieties of hot chilli as a whole with respect to their seed, pericarb and placenta. In fact, the arsenic content found in seed has paid more attention because the arsenic accumulation in seed of plant can be regulated by inositol transporters. In addition, this study leads to improve such mild acid extraction method as a routine work for other metal residues in foods and vegetables with its green emerging applications for future trends.
Acknowledgements
This research was financially supported by the Higher Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Food and Functional Food Research Cluster of Khon Kaen University, and Materials Chemistry Research Center, and Center for Innovation in Chemistry (PERCH-CIC), Commission on Higher Education, Ministry of Education Thailand.
References
- Sricharoen, P.; Techawongstein, S.; Chanthai, S. J. Food Sci. Technol. 2015, 52, 8077-8085.
CrossRef - Sricharoen, P.; Lamaiphan, N.; Patthawaro, P.; Limchoowong, N.; Techawongstien, S; Chanthai, S. Ultrason. Sonochem. 2017, 38, 629-639.
CrossRef - Costa, B.E.D.S.; Coelho, N.M.M.; Coelho, L.M. Food Chem. 2015, 178, 89-95.
CrossRef - Sricharoen, P.; Limchoowong, N.; Areerob, Y.; Nuengmatcha, P.; Techawongstien, S.; Chanthai, S. Ultrason. Sonochem. 2017, 37, 82-92.
CrossRef - Limchoowong, N.; Sricharoen, P.; Areerob, Y.; Nuengmatcha, P.; Sripakdee, T.; Techawongstien, S.; Chanthai, S. Food Chem. 2017, 230, 388-397.
CrossRef - Gupta, J.; Gupta, A.; Gupta A.K. Orient. J. Chem. 2014,30(2), 815-819.
CrossRef - Kumaravel, S.; Alagusundaram, K. Orient. J. Chem. 2014, 30(2), 631-636.
CrossRef - Michael, C.; Sugumar, R.W. Orient. J. Chem. 2013, 29(3), 1149-1154.
CrossRef - Markovski, J.S.; Đokić, V.; Milosavljević, M.; Mitrić, M.; Perić-Grujić, A.A.; Onjia, A.E.; Marinković, A.D. Ultrason. Sonochem. 2014, 21(2), 790-801.
CrossRef - Sigrist, M.; Hilbe, N.; Brusa, L.; Campagnoli, D.; Beldoménico, H. Food Chem. 2016, 210, 96-101.
CrossRef - Rahman Md.F.; Chakraborty, A.; Das, T. Orient. J. Chem. 2015, 31(4), 2401-2408.
CrossRef - Korotkova, T.G.; Ksandopulo, S.J.; Bushumov, S.A. Burlaka, S.D.; Say, Y.V. Orient. J. Chem. 2017, 33(1), 186-198.
- Leong, J.H.; Ong, K.K.; Zin, W.Y.W.M. Orient. J. Chem. 2016, 32(5), 2407-2403.
CrossRef - Sricharoen, P.; Limchoowong, N.; Techawongstien, S.; Chanthai, S. Arabian J. Chem. 2017, https://doi.org/10.1016/j.arabjc.2016.12.020
CrossRef - Limchoowong, N.; Sricharoen, P.; Techawongstien, S.; Chanthai, S. Food Chem. 2016, 200, 223-229.
CrossRef - de Paula, C.E.R.; Caldas, L.F.S.; Brum, D.M.; Cassella, R.J. J. Pharm. Biomed. Anal. 2013, 74, 284-290.
CrossRef - Deng, J.; Feng, X.; Qiu, X. Chem. Eng. J. 2009, 152(1), 177-182.
CrossRef - Limchoowong, N.; Sricharoen, P.; Techawongstien, S.; Chanthai, S. Food Chem. 2017, 230, 398-404.
CrossRef - Limchoowong, N; Tan-Umporn, P.; Laijungred, L.; Techawongstein, S.; Chanthai, S.; Orient. J. Chem. 2015, 31(1), 171-176.
CrossRef - Limchoowong, N.; Sricharoen, P.; Techawongstien, S.; Kongsri, S.; Chanthai, S. J. Braz. Chem. Soc. 2017, 28(4), 540-546.
- Sricharoen, P.; Limchoowong, N.; Techawongstien, S.; Chanthai, S. Food Chem. 2016, 203, 386-393.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









