Structural and Biological Activity Study of (E)-N’-(5-Chloro-2-Hydroxybenzylidene)Nicotinohydrazide) [H2L] and Some of its Divalent Metal Complexes.
Ogunniran Kehinde Olurotimi, Adekoya Joseph Adeyemi, Bamgboye Omolara Agbeke, Ojo Olajumoke, Siyanbola Tolutope Oluwasegun and Ezenkeanyi Godfery Sunday
Department of Chemistry, College of Science and Technology, Covenant University, Ota, Ogun State, Nigeria.
Corresponding Author E-mail: kehinde.ogunniran@covenantuniversity.edu.ng
DOI : http://dx.doi.org/10.13005/ojc/330405
Nicotinic acid hydrazide and 2-hydroxyl-5-chlorobenzaldehyde were condensed at 80oC to form an ONO coordination pattern ligand (H2L). The structure of the ligand was elucidated by using 1H NMR, 13C NMR, COSY, HSQC, CHN analyzer, ESI mass spectrometry and IR, Thereafter, five metal complexes [Cu(II), Fe(II), Zn(II), Mn(II) and Ni(II)] of the ligand were synthesized and their structural elucidation were achieved by using elemental analysis, infrared, EPR, molar conductivity and powder X-ray diffraction studies. Structural geometries of some of the compounds were predicted. Based on the analysis, octahedral geometry was suggested for the complexes. In vitro antitubercular activity study of the ligand and the metal complexes were evaluated against Mycobacterium tuberculosis, H37Rv, by using micro-diluted method. The results obtained (H2L- MIC = 0.86 µg/mL), (CuL- MIC = 0.64 µg/mL), (FeL- MIC = 0.77 µg/mL), (ZnL- MIC = 0.69 µg/mL), (MnL- MIC = 1.12 µg/mL) and (NiL- MIC = 0.92 µg/mL) revealed that all the compounds except (MnL- MIC = 1.12 µg/mL) possess better activity when compared with one of the first line drug (isoniazid) (INH- MIC = 0.9 µg/mL). H2Lexhibited higher antitubercular activity with MIC value of 0.86 µg/mL. However, the metal complexes displayed higher cytoxicity but were found to be non-significant different (P ˂ 0.05) to isoniazid drug.
KEYWORDS:Hydrazones; electron spin resonance; thermogravimetric; powder X-ray diffraction; antitubercular agents
Download this article as:| Copy the following to cite this article: Olurotimi O. K, Adeyemi A. J, Agbeke B. O, Olajumoke O, Oluwasegun S. T, Sunday E. G. Structural and Biological Activity Study of (E)-N’-(5-Chloro-2-Hydroxybenzylidene)Nicotinohydrazide) [H2L] and Some of its Divalent Metal Complexes. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Olurotimi O. K, Adeyemi A. J, Agbeke B. O, Olajumoke O, Oluwasegun S. T, Sunday E. G. Structural and Biological Activity Study of (E)-N’-(5-Chloro-2-Hydroxybenzylidene)Nicotinohydrazide) [H2L] and Some of its Divalent Metal Complexes. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=34360 |
Introduction
Tuberculosis (TB) is measured to be amongst the 10 top causes of death across the globe (WHO, 2016). The TB widespread is greater than formerly estimated. In 2015, about 10.4 million new cases of TB was recorded of which 56% of them are men while 34% are women and 10% are children. In the same year, the number of deaths from multidrug-resistant TB (MDR-TB) and rifampicin-resistant TB (RR-TB) increased by 0.4 million. Tuberculosis resulted to estimate 1.4 million deaths in 2015 worldwide with six countries like India, Indonesia, China, Nigeria, Pakistan and South Africa accounted for 60% of the deaths (WHO, 2015; Ian, 2016). Based on the WHO reports, the need to reduce or eradicate the occurrence of the disease before 2020 is of high priority.
Emerging transition metal complexes with anti-tubercular activity has engrossed substantial interests among the bio-inorganic chemists over the world based on the past success of cisplatin (Chen et al., 2013; Dasari and Tchounwou, 2014). Hydrazones are group of compounds found with numerous biological activities which includes antibacterial, antitumor, antimalarial and antituberculosis (El-Sherif, et al., 2012; Hosney, et al., 2010). The reports (Vogel,et al., 2008; Grandeet al., 2007) have revealed that hydrazides, RCONHNH2 and hydrazones, RCONHN=CHR’, are classes of compounds that are effective as chelating agents with transition metal ions. They have readily available lone pairs of electrons useful for bind with the transition metal ions (Stadler and Harrowfield, 2009). Based on these backgrounds, scientists are now directing their works on substituted hydrazones. Metal complexes of substituted hydrazones have been stated to display therapeutic activities (Angelusiu et al., 2010; Hosney, et al., 2010). The metal complexes bind and cleave the DNA strands. Hence, the compounds possess could be used for biomimetic applications and as models for elucidation the mechanism of enzyme inhibition (Michael, 2010; Jagvir and Prashant, 2012).
In the present work, an arylhydrazone obtained in the reaction of nicotinic hydrazide with 5-chloro-2-hydroxylbenzaldehyde and its Cu(II), Fe(II), Zn(II), Mn(II) and Ni(II) complexes were characterized and tested for their antimycobacterium activity. The presence of the hydrazine pharmacophore in these compounds is expected to contribute to high antimycobacterium activity.
Methods
Chemicals
The reagents and chemicals used in this work were purchased from Sigma-Aldrich. They are analytical grade and used without further purification.
Physical Measurements
The mass spectrum of H2L was obtained on Agilent 6520 Q-TOF mass spectrometer. The % of carbon, nitrogen and hydrogen of the hydrazone and its metal complexes were determined by using Vario EL CHNS analyzer. 1H, 13C and 2D NMR (COSY and HSQC) spectra H2L were recorded by using Bruker AMX 300 FT-NMR spectrometer in DMSO-d6. The IR spectral of the H2L and its metal complexes were recorded on Perkin – Elmer Fourier Transform Infrared Spectrometer using KBr pellets in the range of 4000 – 400 cm-1. The magnetic data of some of the metal complexes were measured at room temperature by using vibrating susceptibility magnometer. TGA/DTA thermographs of some of the metal complexes were obtained by using thermogravimetric analyzer TGA Q500 V20.8 Build 34. The EPR spectra some of the metal complexes recorded at 77 K were recorded on Varian E-112 spectrometer with TCNE used as the standard. Powder X-ray diffraction data for one of the metal complexes was recorded with a Bruker AXS D8 Advance diffractometer.
Synthesis of (E)-N’-(2-Hydroxyl-5-Chlorobenzylidene)Nicotinohydrazide [H2L]
The synthetic methods previously described [Mandewale et al., 2015; Thomas et al., 2011] was modified and adopted. The nicotinic acid hydrazide (10 mmole, 137 mg) was dissolved in 20 ml of absolute ethanol by heating gently on water bath. The solution obtained was mixed with ethanoic solution of 2-hydroxyl-5-chlorobenzaldehyde (10 mmole) in a round bottom flask. The mixture was refluxed stirred at 80oC for 6 h after which it was allowed to stand at ambient temperature for 24 h. The precipitate formed was filtered and washed three times with cold ethanol. The precipitate was recrystallized in mixture of methanol and chloroform (1:1). It was filtered off, washed with ether and dried in vacuum. The reaction was monitored with the use of TLC using methanol: chloroform (2:8) mixture.
 |
Scheme 1 Click here to View scheme |
The aldehyde used in the synthesis is 5-chloro-2-hydroxylbenzaldehyde; yield 1.76 g (71.3 %); mp: 229-230 oC; Rf = 0.82 (CHCl3/CH3OH, 4:1, at RT.). 1H-NMR (DMSO-d6) δ: 12.36(s, 1H, NH), 11.21 (s, Ar-OH, 1H), 9.13 (d, J = 1.71 Hz, 1H, H(3)), 8.83 (dd, J1 = 1.2 Hz, J2 = 4.7 Hz, 1H, H(4)), 8.67 (s, 1H, H-C=N), 8.33 (dt, J1 = 1.71 Hz, J2 = 6.15 Hz ,1H, H(6)), 7.74 (d, J= 2.61 Hz, 1H, H(7)), 7.64 ( m, J1 = 4.83 Hz, J2 = 7.86 Hz, 1H, H(8)), 7.39 (dd, J1 = 2.64 Hz, J2 = 8.64 Hz, 1H, H(9)) , 7.02 (d, J= 8.76 Hz, 1H, H(10)), ppm. 13C-NMR (DMSO-d6) δ: 162.12 (CO), 156.56 (C-OH), 152.99 (C(3)), 149.17 (C(4)), 146.76 (HC=N), 136.0 (C(6)), 131.48 (C(7)), 129.13 (C(8)), 127.98 (C(9)), 124.14 (C(10)), 123.58 (C(11)), 121.18 (C(12)) 118.5 C(13)), ppm. IR (KBr) cm-1: 3502-3328 (ArOH), 3193 (NH), 1666 (C=O), 1613 (C=N), 1552 (N-N), 1347 (C-O), 1158 (C-N), 707 (C-Cl). MS (ESI+): in m/z: 276.2 [M + H]+. Anal. calcd. for C13H10ClN3O2 (275.05): C, 57.72; H, 3.64; N, 15.27. Found: C, 57.55; H, 3.83; N, 14.88.
Synthesis of Metal Complexes
Synthesis of Cu(II) Complexes of (E)-N’-(5-Chloro-Dihydroxybenzylidene)Nicotinohydrazide [Cu(H2L)2]SO4.
Ethanolic solution of H2L was prepared by dissolving 20 mmol of H2Lin 15 mL of absolute ethanol in a round bottomed flask. The solution was warmed to 50oC on a water bath for 40 min before adding 15 mL of ethanolic solution (10 mmol) of CuSO4.The mixture was refluxed at 80oC for 4 h. The green product formed was filtered after cooling the solution in iced water, washed with ethanol and ether, and then dried over P4O10 in vacuo. Yield: 187 mg (84.61%).
Elemental Anal. found (Calcd.) (%): Cu, 8.86 (8.94); C, 43.85 (43.92); H, 3.07 (2.84); N, 11.54 (11.82). μ= 2.1 BM.
Synthesis of Fe(II) Complexes of (E)-N’-(5-Chloro-2-Dihydroxybenzylidene)Nicotinohydrazide [Fe(H2L)Cl2]
Fe(III) complex of H2L was prepared by dissolving 161 mg (10 mmol) of H2Lin 10 ml of absolute ethanol in a round bottomed flask. The solution was with ethanolic solution of FeCl2. The mixture was refluxed for 4 h and kept at room temperature for 4 days to obtain a deep brown product which was with cold ether and dried over P4O10 in vacuo. Yield: 128 mg (55.89%).
Elemental Anal. found (Calcd.) (%): Fe, 15.65 (15.22); C, 43.25 (42.55); H, 3.07 (2.75); N, 11.17 (11.45). μ= 5.90 BM.
Synthesis of Zn(II) Complexes of (E)-N’-(5-Chloro-2-Dihydroxybenzylidene)Nicotinohydrazide [Zn(H2L)2]
10 mmol (229 mg) of Zn(CH3COO)2 was dissolved in 10 mL of absolute ethanol , The solution obtained was added gradually to ethanolic solution of H2L (10 mmol) in a round bottomed flask after which two drops of triethylamine (TEA) was added. The solution was refluxed for 3 h and the yellow precipitate obtained was dried over P4O10 in vacuo after washing with cold ethanol. Yield: 152 mg (78.76%).
Elemental Anal. found (Calcd.) (%): Zn, 10.52 (10.60); C, 50.78 (50.63); H, 3.49 (3.27); N, 13.87 (13.63). μ= 0 BM.
Synthesis of Mn(Ii) Complexes Of (E)-N’-(5-Chloro-2-Dihydroxybenzylidene)Nicotinohydrazide [Mn(H2l)2]H2o
10 mmol (161 mg) of H2L dissolved in 10 ml of absolute ethanol was added to 10 mmol of Mn(CH3COO)2.4H2O in a round bottomed flask. The mixture was refluxed for 4 h after adding two drops of TEA. The brown precipitate obtained was dried over P4O10 in vacuo after washing with cold ethanol Yield: 173 mg (53.2%).
Elemental Anal. found (Calcd.) (%): Mn, 8.28 (8.80); C, 49.63 (50.02); H, 3.67 (3.55); N, 13.05 (13.46). μ= 6.03 BM.
Synthesis of Ni(II) Complexes Of (E)-N’-(5-Chloro-2-Dihydroxybenzylidene)Nicotinohydrazide [Ni(H2L)2]
10 mmol of NiCl2.4H2O was dissolved in 10 ml of ethanol and was added to ethanolic solution of H2L(20 mmol in 10 ml of ethanol) in a round bottomed flask. The solution was refluxed for 4 h and the precipitate formed was allowed to stand at ambient temperature for 12 h after which it was filtered, washed and dried over P4O10 in vacuo. Yield: 196 mg (52.10%).
Elemental Anal. found (Calcd.) (%): Ni, 9.23, (9.34); C, 50.60 (51.19); H, 3.57 (3.30); N, 13.55 (13.78). μ= 2.92 BM.
Antimycobacterial Activity Study
Results and Discussion
NMR Spectral Studies of (E)-N’-(2-Hydroxyl-5-Chlorobenzylidene)Nicotinohydrazide [H2L]
1H NMR
1H NMR spectrum of H2L (Figure 1.0) in DMSO-d6 showed singlet peaks at δ = 12.36 ppm and δ = 11.21 ppm. They were assigned to NH(1) and OH(2) proton respectively. Both peaks were strongly de-shielded due to direct attachment to high electronegative atoms (Cui et al., 2012; Webster and Silverstein, 2006). The doublet signal at δ = 9.13 ppm, and doublet of doublet signal at δ = 8.83 ppm were attributed to H(3) and H(4) respectively. Both peaks appeared downfield of TMS as expected because of the de-shielding effect from the adjacent N(1) atom. Also, the singlet peak at δ = 8.67 ppm was assigned to H(5) proton. The lower field δ value observed was attributed to conjugative effect from adjacent N(3)-N(2)-C(1) = O(1) core of the hydrazone. The H(6) and H(8) protons in the pyridyl ring, were assigned to the signal at δ = 8.33 ppm and δ = 7.64 ppm respectively. H(6) appeared as doublet of triplet while H(8) appeared as a multiplet signal. In the phenol ring, the doublet peak that integrated as one proton at δ = 7.74 ppm corresponded to H(7) protons while the H(9) and H(10) protons were assigned to doublet of doublet peak at δ = 7.39 ppm and to a doublet peak at δ = 7.02 ppm respectively.
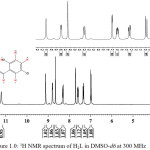 |
Figure 1: 1H NMR spectrum of H2L in DMSO-d6at 300 MHz |
Cosy
The assignments of proton was confirm with the use of 1H-1H correlation spectroscopy (Figure 2.0). The cross peaks along both sides of the diagonal identify the proton that are coupled to each other (Webster and Silverstein, 2006; Benjamin and Dell, 2008). The proton at 12.36, 11.21 and 8.67 ppm assigned to H(1), H(2) and H(5) proton did not show correlation in the COSY spectrum. Therefore, the observation accounted for singlet signals observed in the spectrum. In the pyridyl ring, the signal at 9.13 ppm, assigned to H(3) showed a weak correlation with H(6) at 8.33 ppm and at the same time, H(6) also correlated with H(8), while H(8) showed direct correlation with both H(6) and H(4). Based on this, the signal for H(3), appeared as a doublet, while that of H(6) appeared as a doublet of triplet. Also the signal for H(4) appeared as a doublet of doublet while the signal for H(8) appeared as quartet. In the phenol ring region, the signal at 7.39 ppm assigned to H(9) proton experienced ortho-coupling from H(10) and metal-coupling from H(7). The correlation accounted for doublet peaks at 7.74 and 7.02 ppm assigned to H(7) and H(10) respectively. The correlations observed in the COSY spectrum are supported by the J values of each of the protons.
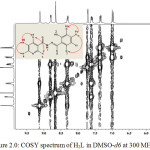 |
Figure 2: COSY spectrum of H2L in DMSO-d6 at 300 MHz Click here to View figure |
13C NMR
The carbon skeleton of the hydrazone was identified by recording its 13C NMR spectrum (Figure 3.0). From the spectrum, it was observed that the carbonyl carbon C(1) resonated at 162.12 ppm while the aromatic carbon C(2) resonated at 156.57 ppm. The two carbons are the most de-shielded carbon due to delocalization of π-electron in the carbonyl and the presence of hydrogen bonding in the hydroxyl group, which reduced the electron density around the carbon atoms (Benjamin and Dell, 2008; Kate et al., 2016). The signals at 152.99 and 149.17 ppm were assigned to C(3) and C(4) respectively. The two carbons resonated downfield due to the high electronegative effect of the adjacent N(1) atom. The resonances at 136.00, 129.13 and 124.14 ppm were assigned to the other pyridyl carbons i.e. C(6), C(8) and C(10) respectively. Azomethine carbon C(5) resonated at 146.76 ppm while the δ values assigned to the other carbons in the phenol ring are C(7), 131.48; C(9), 127.98; C(11), 123.58; C(12), 121.18 and C(13), 118.77 ppm. The two carbons, C(7) and C(9), adjacent to C(12)-Cl resonated at lower field than the other phenol carbons due to conjugative effect.
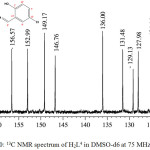 |
Figure 3: 13C NMR spectrum of H2L4 in DMSO-d6at 75 MHz Click here to View figure |
1H-13C HSQC
The 1H-13C HSQC correlation spectrum for the hydrazine (Figure 4.0) was recorded to ascertain the 1H NMR and 13C NMR assignments. HSQC contour was not observed for carbon atoms at 162.12, 156.57, 129.13, 123.58 and 121.18 ppm. These signals were assigned to C(1), C(2), C(8), C(11) and C(12) respectively. Both C(8) and C(11) are quartenary carbons (Webster and Silverstein, 2006; Kate et al., 2016). HSQC spectrum shows eight contours for protonated carbon as expected. Each contour confirmed 1H NMR assignments in correlation with the 13C NMR assignments.
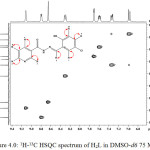 |
Figure 4: 1H-13C HSQC spectrum of H2L in DMSO-d6 75 MHz Click here to View figure |
Physical Properties of Cu(II), Fe(II), Zn(II), Mn(II) and Ni(II) Complexes of (E)-N’-(2-Hydroxy-5-Chlorobenzylidene)Nicotinohydrazide [H2L]
The metal complexes of H2L are of various colours. All the metal complexes precipitated as amorphous powder. The metal complexes were found to possess high melting point (>300 oC). They are sparingly soluble in methanol, chloroform and DMF but are soluble in DMSO and pyridine. The elemental analysis data obtained are in agreement with the theoretical values obtained. The results obtained, combined with other analytical data were used to arrive at the molecular formulae presented in Table (1.0). The magnetic susceptibility data recorded at ambient temperature for Cu(II), Fe(II), Mn(II) and Ni(II) complexes demonstrated full agreement with the calculated spin only magnetic moments for the complexes. As expected, Zn(II) complex was found to be diamagnetic. Low conductance values obtained using conductivity meter for all the complexes in DMSO demonstrated non – electrolytic character of the complexes.
Table 1.0: Analytical data of H2L4 and its metal complexes.
|
Complex |
Colour |
M. pt. oC |
Found (Calc.), % |
LM |
μeff |
|||
|
M |
C |
H |
N |
S cm2 mol-1 |
μB |
|||
| [Cu(H2L)2]SO4 |
Green |
˃300 |
8.86 (8.96) |
43.85 (44.05) |
3.07 (2.56) |
11.54 (11.85) |
43 |
2.1 (1.73) |
| [Fe(H2L)Cl3] |
Deep brown |
˃300 |
15.65 (15.26) |
43.25 (42.66) |
3.07 (2.48) |
11.17 (11.48) |
52 |
5.90 (4.92) |
| [Zn(H2L)2] |
Yellow |
˃300 |
10.52 (10.64) |
50.78 (50.80) |
3.49 (2.95) |
13.87 (13.67) |
45 |
–
|
| [Mn(H2L)2]H2O |
Brown |
˃300 |
8.28 (8.77) |
49.63 (49.86) |
3.67 (3.86) |
13.05 (13.42) |
48 |
6.03 (5.92) |
| [Ni(H2L)2] |
Yellow |
˃300 |
9.23 (9.59) |
50.20 (51.02) |
3.57 (3.62) |
13.55 (13.73) |
46 |
2.92 (2.83) |
H2L = C13H10ClN3O2
Infrared Spectra
The infrared spectral data of the ligands H2L and its metal complexes are presented in Table 2.0. The broad peak between 3328 and 3193 cm-1 attributed to the ν(C-OH) vibrational stretching mode (Stuart, 2004; Webster and Silverstein, 2006) in the hydrazone spectrum was observed as strong and broad peak at higher wavenumbers between of 3413 and 3425 cm-1 in the metal complexes. The observation supported the bonding of the OH group to metal ions in the complexes. The conjugation effect also resulted to the shifting of ν(C-O) deformation mode to higher wavenumber in the complexes.
The strong peak observed at 1666 cm-1 in the spectrum of the free ligand was attributed to the characteristics of ν(C=O) vibrational stretching mode (Stuart, 2004). Similar peak was observed at lower wavelength in the spectral of the complexes except in Ni(II) complex due to effect of ring restrain that prevent free vibration of the chromophoric group after coordination to the metal ion. However, in Ni(II) complex, the band appeared at higher wavenumber with reduction in intensity which indicates formation of covalent bond between the oxygen atom of the carbonyl and Ni(II) ion in the complex. This is further supported by the significant change in the wavelength of ν(C-O) deformation bands which shifted to higher wavenumber in the spectral of the complexes due to the coordination of CO to the metal ion.
The azomethine ν(C=N) vibrational stretching mode at 1613 cm-1 in the spectrum have undergone hyspchromic shift in the metal complexes due to restrain in its vibrational frequency after coordination to metal ions (Shi et al., 2009; Dhanaraj and Nair). The observation is further supported by the higher wavenumber shift for the δ(C-N) deformation bands in the metal complexes. However, the medium peak at 3502 cm-1, which is characteristic of secondary amine of the amide group in the hydrazone, disappeared in the spectra of the metal complexes due to the conjugate effect of the coordination to the azomethine N atom. Other bands observed in the complexes in the regions 518 – 597 cm-1 and 515 – 427 cm-1 are due to ν(M-O) and ν(M-N) respectively.
Table 2.0: Infrared data of H2Land its metal complexes.
| Ligand/Complex | ν(OH)cm-1 | ν(C=O)cm-1 | ν(C=N)cm-1 | ν(N-N)cm-1 | δ(C-O)cm-1 | δ(C-N)cm-1 | ν(M-O)cm-1 | ν(M-N)cm-1 |
| H2L | 3328-3193 m | 1666 s | 1613 m | 1552 s | 1345 m | 1158 m | – | – |
| [Cu(H2L)2]SO4 | 3413 s,b | 1616m | 1523 m | 1456 m | 1371 w | 1179 m | 597w | 515 w |
| [Fe(H2L)Cl3] | 3425 s,b | 1605 m | 1531 w | 1454 w | 1379 w | 1183 m | 559 w | 427 m |
| [Zn(H2L)2] | 3418 s,b | 1614 m | 1528 m | 1470 m | 1377w | 1188 m | 559 w | 510 w |
| [Mn(H2L)2]H2O | 3424 s,b | 1610m | 1560 w | 1455 m | 1377 m | 1177 m | 612 m | 427 w |
| [Ni(H2L)2] | 3420 s,b | 1667 w | 1608 m | 1553 m | 1374 w | 1182 m | 518 w | 497 w |
TGA / DTA Analyses
Thermal analysis curves (TGA / DTA) for Cu(II), Fe(II) and Zn(II) complexes obtained under inert atmosphere within the temperature range of ambient temperature to 700oC are as shown in Figure 5.0. The thermal decomposition of Cu(II) complex proceeded in four degradation steps. The first three degradation steps ranging from 58 – 65, 95 – 115 and 224 – 246oC corresponded to the loss of the SO42- ion from the complex (Gabbott, 2008). The degradation accounted for 14% (Calcd. 13%) loss in the total weight of the complex. The coordinated ligand decomposed to residue within the temperature range of 246 – 450oC. This decomposition corresponded to the loss of 62% (Cald. 61%) of the complex. The residue, which is about 24% (Calcd 23%) of the complex, was considered to be metal oxide and some carbon residue.
The TGA / DTA curve for Fe(III) complex showed one main degradation step between 209 and 539oC which correlated with the decomposition of the ligand in the complex and it was found experimentally to be 65% (Calcd. 66%) weight loss. However, the decomposition observed between 160 and 209oC, equivalent of 18% weight loss, was attributed to loss of coordinated chlorine atoms in the complex. The residue, which is mainly metal oxide, was found to be equivalent to 16% (Calcd. 16.5%) of the complex.
The thermogram for Zn(II) complex showed one main decomposition step which corresponded to 75% (Calcd. 77%) weight loss. This was attributed to the decomposition of the coordinated ligand, H2L. However, the slight decomposition at 183oC and 346oC were considered to be part of the ligand decomposition. The residue, which was mainly metallic oxide, accounted for 25% (Calcd. 23%) of the complex.
![Figure 5.0: TGA / DTA spectra of (a); [Cu(H2L)2]SO4 (b); [Fe(H2L)Cl3] and (c); [Zn(H2L)2]](http://www.orientjchem.org/wp-content/uploads/2017/07/Vol33No4_Stru_Ogun_fig5-150x150.jpg) |
Figure 5: TGA / DTA spectra of (a); [Cu(H2L)2]SO4 (b); [Fe(H2L)Cl3] and (c); [Zn(H2L)2] Click here to View figure |
Electron Paramagnetic Resonance Spectra
The EPR spectra for representative [Mn(II) and Cu(II)] complexes recorded in DMSO at 77 K are as shown in Figures 6.0. The epr spectrum of Mn(II) complex appeared as a broad peak with no hyperfine splitting. However, the giso observed (2.0549) was found to be greater than free electron value of 2.0023. Also, the observed g|| = 3.6193 was found to be greater than g⊥ of 1.9597. The observation suggests that Mn(II) is in octahedral environment (Pilbrow, 1990).
The spectrum of the Cu(II) complex at 77 K showed one intense absorption band at the high field and was isotropic due to the tumbling motion of the molecules. The Cu(II) complex exhibited the g|| value of 2.5921 and g⊥ value of 2.0771. These values suggests a distorted octahedral structure, thus ruling out the possibility of a triagonal bipyramidal structure which would be expected to have g⊥ > g|| .
![Figure 6.0: EPR spectrum of (a); [Mn(H2L)2]H2O and (b); [Cu(H2L)2]SO4 in DMSO at 77 K](http://www.orientjchem.org/wp-content/uploads/2017/07/Vol33No4_Stru_Ogun_fig6-150x150.jpg) |
Figure 6: EPR spectrum of (a); [Mn(H2L)2]H2O and (b); [Cu(H2L)2]SO4 in DMSO at 77 K Click here to View figure |
Powder X-Ray Diffraction Studies
The crystal lattice parameters for Cu(II) complex were measured by using a Bruker AXS D8 Advance diffractometer. An X-ray diffractogram was recorded (Figure 7.0) in the range of 5º to 120º on 2θ scale. The major peaks of relative intensity greater than 10% were indexed to yield the Miller indices (hkl), the unit cell parameters and the unit cell volume. The unit cell parameters of Cu(II) complex yielded values of lattice constants: a = 21.315 Å, b = 25.031 Å and c = 10.045 Å and a unit cell volume V of 5359.36 Å3. The parameters were found to be in agreement with orthorhombic lattice system in which the conditions such as a ≠ b ≠ c and α = β = γ = 90˚ were satisfied. Therefore, [Cu(H2L)2]SO4 complexes was considered to have a distorted octahedral geometry (Shoemaker and Garland, 1989).
![Figure 7.0: X-ray diffraction pattern of [Cu(H2L)2]SO4](http://www.orientjchem.org/wp-content/uploads/2017/07/Vol33No4_Stru_Ogun_fig7-150x150.jpg) |
Figure 7: X-ray diffraction pattern of [Cu(H2L)2]SO4
|
Based on the analytical data and spectroscopic data obtained, the computer model structures of the complexes are shown below:
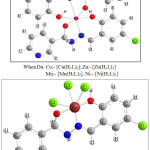 |
Figure 8: Proposed structures for the complexes |
Anti-Mycobacterial Study
The anti-mycobacterial properties of the H2L ligand and its corresponding metal complexes were established by determining their minimum inhibitory concentration (MIC) represented in Table and Figure 8.0. The results indicated that Cu(II), Fe(III) and Zn(II) complexes are more potent than the standard antibiotic (isoniazid) with MIC values of 0.64, 0.77 and 0.69 μg/mL respectively. This indicates that the metal ions play significant role in inhibitory activities of the complexes (Anna et al., 2009; Petra, et al., 2003; Koen, et al., 2006). However, Mn(II) and Ni(iI) complexes were found to possess moderate inhibitory activity as compare to isoniazid. The two complexes show lesser activity than the standard antibiotic. Generally, the complexes demonstrated higher activity against drug-resistant strains of Mycobacterial Tuberculosis. However, they were found to be toxic to Vero cell as indicated by their IC50 values (Table 3.0).
Table 3.0: MIC (µg/mL), IC50 (µM) and SI (IC50/MIC) values of the metal complexes of H2L.
|
Compound Code |
MIC (µg/ml) |
IC50 (µM) |
SI (IC50/MIC) |
|
CuL |
0.64 ± 0.073 | 1.52 |
2.37 |
|
FeL |
0.77 ± 0.056 | 2.09 |
2.71 |
|
ZnL |
0.69 ± 0.022 | 1.13 |
1.64 |
|
MnL |
1.12 ± 0.159 | 1.21 |
1.08 |
|
NiL |
0.92 ± 0.271 | 1.25 |
1.36 |
|
H2L |
0.86 ± 0.201 | 2.22 |
2.58 |
INH (Control) = 0.91 ± 0.133 (µg/mL)
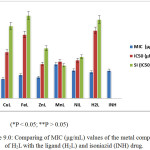 |
Figure 9: Comparing of MIC (μg/mL) values of the metal complexes of H2Lwith the ligand (H2L) and isoniazid (INH) drug. Click here to View figure |
Conclusion
(E)-N’-(5-chloro-2-hydroxybenzylidene)nicotinohydrazide) ligand and five of its transition metal complexes were synthesized with good yields under simple reflux conditions.. The structures of the desired complexes were determined by spectral, thermal and powder xrd studies. The ligand coordinated to the transition metal in an octahedral mode forming M-ONO. The powder X-ray diffraction data for [Cu(H2L)2]SO4 complex further supported the geometry of the complex. The
Complexes, except MnL and NiL, revealed higher activity against drug-resistant strains of Mycobacterial Tuberculosis indicating that the compounds are potential antitubercular agents.
Acknowledgements
The authors acknowledge the support of TWAS and CSIR for the award of fellowship for this study.
References
- Angelusiu, M. V., Barbuceanu, S. F., Draghici, C. Almajan, G. L.,. , New Cu(II), Co(II),Ni(II) complexes with aroyl-hydrazone based ligand. Synthesis, spectroscopic characterization and in vitro antibacterial evaluation. Eur. J. Med. Chem. 2010, 45, 2055-2062.
CrossRef - Anna, C. H., Rooda, A., Marijke, J. W., Klaas, K. B., Karen, V., Jerome, G., Koen, A., Holger, L., Anil, K., Dirk, B.,. ) Selectivity of TMC 207 towards Mycobacterial ATP Synthase Compared with That towards the Eukaryotic Homologue, Antimicrob. Agents Chemother., 2009., 53 (3), 1290-1292.
CrossRef - Benjamin, C., Dell, J.,. Synthesis and Characterization of 9-Hydroxyphenalenone Using 2D NMR Techniques, J. Chem. Educ., 2008.,85 (3), DOI: 10.1021/ed085p413.
CrossRef - Cui, Y., Dong, X., Li, Y., Chen, W., Synthesis, structures and urease inhibition studies of Schiff base metal complexes derived from 3, 5-dibromosalicylaldehyde, Eur. J. Med. Chem., 2012, 58, 323-331.
CrossRef - Dasari, S., Tchounwou, P.B.,. Cisplatin in cancer therapy: molecular mechanisms of action, Eur. J. Pharmacol., 2014.,740, 364–378.
CrossRef - Dhanaraj, C.J., Nair, M. S.,. Synthesis and characterization of metal(II) complexes of poly(3-nitrobenzylidene-1- naphthylamineco-succinicanhydride) Eur. Polym. J. 2009., 45, 565–572.
CrossRef - El-Sherif, A. A., Shoukry, M.M., Mohamed, M. A., Synthesis, characterization, biological activity and equilibrium studies of metal(II) ion complexes with tridentate hydrazone ligand derived from hydralazine, Spectrochim. Acta A., 2012. .,98, 307-321.
CrossRef - Erno, P., Philippe, B., Martin, B.,. Structure Determination of Organic Compounds, 4th Ed., Springer-Verlag Berlin Heidelberg, 2009.,10-187.
- Grande, F., Aiello, F. De Grazia, O., Brizzi, A., Garofalo, A., Neamati, N.,. Synthesis and antitumor activities of a series of novel quinoxalinehydrazides. Bioorg. Med. Chem., 2007., 15, 288-294.
CrossRef - Ian, M. O., Vaccines to prevent tuberculosis infection rather than disease: Physiological and immunological aspects, Tuberculosis, 2016, 101, 210-216.
CrossRef - Jagvir, S., Prashant, S.,. Synthesis, Spectroscopic Characterization, and In Vitro Antimicrobial Studies of Pyridine-2-Carboxylic Acid N -(4-Chloro-Benzoyl)-Hydrazide and Its Co(II), Ni(II), and Cu(II) Complexes, Bioinorg. Chem. Appl. 104549, doi:10.1155/2012/104549. 2012.
CrossRef - Kate, J. G., Edward, J. M., Chris, P. S.,. Web-Based 2D NMR Spectroscopy Practice Problems, J. Chem. Edu. 2016., 93 (8), 1483-1485.
CrossRef - Koen A., K., Verhasselt, P., Guillemont, J., Göhlmann, H. W. H., Jean-Marc, N., Winkler, H., Gestel, J. V., Timmerman, P., Zhu, M., Ennis, L., Williams, P., Chaffoy, D., Huitric, E., Hoffner, S., Cambau, E., Truffot-Pernot, C., Lounis, N., Vincent Jarlier, V., A Diarylquinoline Drug Active on the ATP Synthase of Mycobacterium tuberculosis, Science, Science, 2005. 307, (5707), 223-227, DOI: 10.1126/science.1106753.
CrossRef - Mandewale, M. C., Thorat, B. R., Shelke, D., Yamgar, R. S. 2015, Synthesis and biological evaluation of new hydrazone derivatives of quinoline and their Cu(II) and Zn(II) complexes against Mycobacterium tuberculosis,Bioinorg. Chem. doi.org/10.1155/2015/153015.
CrossRef - Michael R. L.,. The Mechanism of Double-Strand DNA Break Repair by the Nonhomologous DNA End Joining Pathway, Annu. Rev. Biochem. 2010.,79, 181–211.
CrossRef - Paul G.,. , Principles and Applications of Thermal Analysis, 1st Ed., John Wiley & Sons 2008.
- Petra, L., Edda, B., Elke, G., Jutta, W., Helmut, H., Comparison of Broth Microdilution, E Test, and Agar Dilution Methods for Antibiotic Susceptibility Testing of Campylobacter jejuni and Campylobacter coli, J. Clin. Microb., 2003. 41( 3), 1062-1068.
CrossRef - Pilbrow, J. R.,. Transition Ion Electron Paramagnetic Resonance, Clarendon Press Oxford, Clarendon Press Oxford, 1990.,12 – 89.
- Shi, L., Mao, J., Yang, Y., Zhu, H. L.,. “Synthesis, characterization, and biological activity of a Schiff-base Zn (II) complex,” J. Coord. Chem., 2009,. 62 (21), 3471–3477.
CrossRef - Shoemaker, D. P., Garland, C. W., Experiments in Physical Chemistry, 5th Ed., Mc Graw-Hill, International Edition, New York, 1989. 2- 234.
- Stadler, A.M., Harrowfield, J., Bis-acyl-/aroyl-hydrazones as multidentate ligands. Inorg. Chim. Acta, 2009, 362, 4298-4314.
CrossRef - Stuart, B. J.,. Infrared spectroscopy fundamentals and applications; Wiley, Chichester, Eng. Hoboken, N.J. 2004 .,1-122.
- Sui, M., Matthew, T., Mauger, L., N., Jonathan, B. B., Solon, K., Brian, E. B., Jun-qi, L., Franklin, R. T.,. Potential applications of antimicrobial peptides and their mimics in combating caries and pulpal infections, Acta Biomaterialia, 2017., 49, 16–35.
CrossRef - Thomas, K. D., Adhikari, A. V. Telkar, S. Chowdhury, I. H. Mahmood, R., Pal, N. K., 2011. Design, synthesis and docking studies of new quinoline-3-carbohydrazide derivatives as antitubercular agents, Eur. J. Med. Chem. 2017.,46, 5283–5292.
CrossRef - Vogel, S., Kaufmanna, D., Pojarováa, M., Müller, C., Pfaller, T., Kühne, S., Bednarski, P. J., Angerer, E.V., Aroyl hydrazones of 2-phenylindole-3-carbaldehydes as novel antimitotic agents. Bioorgan. Med. Chem., 2008.,16, 6436-6447.
CrossRef - Webster, F. X., Silverstein, R. M.,. Spectrometric Identification of Organic Compounds, 6th Ed., Wiley –India, 2006.,1-216.
- World Health Organization, , Global status report on non-communicable diseases. 2015., 1-161.
- World Health Organisation, , Global Tuberculosis Report. 2015.
- World Health Organisation, 2016, Global Tuberculosis Report, http://reliefweb.int/report/world/global-tuberculosis-report-2016.
- Zhen-Feng, C., Yun-Qiong, G., Xiao-Yan, S., Yan-Cheng, L., Yan, P., Hong, L., Synthesis, crystal structure, cytotoxicity and DNA interaction of 5,7-dichloro-8-quinolinolato-lanthanides, Eur. J. Med. Chem. 2013..59, 194-202.

This work is licensed under a Creative Commons Attribution 4.0 International License.









