S-NICS Investigation for Heterocyclic Anticancer Compounds
Nayer Mohammadian, Karim Zare and Majid Monajjemi
Department of Chemistry, Science and research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: m_monajjemi@srbiau.ac.ir
DOI : http://dx.doi.org/10.13005/ojc/330402
There are no works in theoretical of a statistical methodof NICS-nucleus independent chemical shift for study of heterocyclic rings, since the asymmetry15(η)and skew15(κ) parameter is fluctuated in small distance and are alternate in large distance in the center of heterocyclic rings.Through changing the asymmetriesamong 0 ≤η≤+1 skew15could be changed in the range of -1 ≤κ ≤+1 , and also the parameter κ will be zero when σ22 = σiso15.In position of axially-symmetric tensors, σ22 equals15 either σ11 or σ33, skewsare κ=±1. In this study, we have investigated the statistical methodsthrough nucleus-independent chemical shifts-SNICS calculations in view point of Bq motions in the center of sphere spaces of heterocyclic rings. In our previous work15, it has been exhibited that S-NICS method is an accurate method for estimation the amount of aromaticity in the non-benzene15 rings similar heterocyclic rings which are popular moleculesin organic chemical compounds as anti-cancer disease.Although NICS values for benzene and naphthalene and so on can be indicated as aromaticity criterion, for other molecules such as heterocyclic rings and their derivatives, S-NICS values are much more accurate compare to NICS index.
KEYWORDS:S-NICS; NICS; heterocyclic anticancer compounds
Download this article as:| Copy the following to cite this article: Mohammadian N, Zare K, Monajjemi M. S-NICS Investigation for Heterocyclic Anticancer Compounds. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Mohammadian N, Zare K, Monajjemi M. S-NICS Investigation for Heterocyclic Anticancer Compounds. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=34977 |
ntroduction
Cancer is a disease dating as far back as the dinosaurs has found cancerous lesions on dinosaur bones. Egyptians also drew examples ofbreast cancer in their hieroglyphics on papyrus, and by the 4th century B.C. manytypes of tumors such as stomach and uterine cancer had been described. In 1775 Percival Pott, a London physician, linked the incidence of scrotal cancer inmen to their jobs as chimney sweeps when they were young boys. Itwas not,however, until the 19th century that scientists began to study cancer systematically,looking at what causes cancer and how it can be cured.
Cancer is one of the important causes of death in the new century. This work is done for developing of modern anticancer drugs. Many of heterocyclic compounds are known as anticancer drugs such as alkylating agents which have targeted cell DNA causing cell death.
Heterocycles1structures are composed by atoms other than carbon, where the most times substituents are sulfur, oxygen1 and nitrogen1,2 .The model size of heterocycles ring, together with the substituent group of the core scaffold2, impact tightly on the chemical and physical properties3 while among the clinical applications, heterocyclic compound has an active role as anti-bacterial4,5, anti-viral6 , anti-fungal7 , anti-inflammatory8 and anti-tumor drugs9-11.
Among the heterocyclic compounds the pyridine, thiophene and Thiazole derivatives exhibited large amount cytotoxicity towards the cancer cell lines. Structure activities relationship was reduced9 from biological results and will be used in further design11 of new active compounds. Currently, a number of drugs are used in the treatment11 of the cancer, but most of them were produced controlled effect on the cancer cells. The usual applications of heterocycles are as vast as it is diverse and is not extensively9 encompassed in the scope10 of that study.
The most drugs belong to a class of hetero-genius structures. Heterocyclic structures played an important behavior in the metabolism11 of all cells; maximum number of them is 6 (or sometimes 5) membered hetero-cycles including one to three heteroatoms11. Recently, imidazole fragment has been attracting much concentration because of its role as attractive scaffold for biochemical active heterocyclic11drugs12.
Generally, chemical-physics and biochemical properties like acceptor and donor capabilities, hydrogen bond, π-π interactions, van der Waals, coordination12 bond with a metal and in total hydrophobic force has caused much interest in anticancer studies for such compounds. These properties are important of understanding for its reactivity enable derivative for binding with various nucleic acids, enzymes and biological structures13,14.
A large number of the important heterocyclic compounds are used in the medical activities such as histidine and prolinewhich are amino acids. It is notable pyridoxine, folic acid, thiamine, riboflavin, biotin, B12 and E families of the vitamins are included of heterocyclic structures. For investigation of antifungal activity compounds, Singh et al have synthesized 1,3,4oxadiazolo-(3,2a)-s-triazin-7-thione13,14 and Abdle,et-al have synthesized some novel 1, 3, 4oxadiazole derivatives12,13. Fungi13 are hetero-tropic micro-organisms that are distinguished13.
Dharhas synthesized 1,3,4-oxadiazolo-[3,2-a]-1,3,4-dithiazinesand found anti-fungal.In compound Ar=2- ClC6H4, Ar’=2- ClC6H4OCH2.Methyl 5-( l-hydroxy-2-propenyl)-3-thiophenecarboxylate was stirred at roomtemperature with 10 equivalents of freshly prepared manganese dioxide to give methyl 5-(2-propenoyl)-3- thiophenecarboxylatein 60% yield.The proton nmr spectrum exhibit explicitly a doublets for two hydrogens due to the de-shielding effect of the carbonyl. Infra-red spectroscopy helps to confirm the structure of two carbonyls around 1650 cm-1 for the allylic ketone and 1700 cm-1 for the ester Figs1-3.
Since a carcinogen is applied into a body, cancerous cell will not immediately result. This is due to the “latency effect” where certain of time elapses before there is growth of the tumor. The initial application of a carcinogen will result in the formation of the irreversible initiated cells. Time may then elapse before a second agent, known as the promoter, will act reversibly on the initiated cell giving a premalignant lesion. Changes in the premalignant lesion, such as increased growth rate, increased invasiveness and metastases, result from the 3rd stage of the process known as progression. Those changes are usually associated with the changing in the number and arrangement of genes which encode for various proteins.
Theoretical Background
Aaromaticity in point of nucleus-independent chemical shifts, with NICS (0), at the center of ring plane were compared in several studiesin long distances. In small range of distance a few works have been done in theoretical and reports the statistical approach in our works15
For anyfurther discussion of statistical methods in S-NICS especially in short range of distances,weexhibited that the asymmetry(η)15and skew(κ)15 fluctuate around the center of rings. The maximum fluctuations15 are visible around the extremums functions mathematically15.
The fundamental of this work is based on random motions of dummy atom in de-shielding spaces of heterocyclic ringsfor consideringthe most abundant of points. The majour purpose of random data of several probes inside of de-shielding spacesis for understanding of anisotropic spin–spin interaction in short distances.
In this study, the major components16-18of Haeberlen17 parameters16-18, has been calculated for heterocyclic rings. A large number of random points near to the center of those rings have been generated by pseudo-random numbers15 generation, which is distributed in a Gaussian function between the interval [0, 1)15.
The results have been compared through the energy-decomposition-analyzation (EDA).The bond energy and conjugation of between heteroatoms bonds of ringsare significantly accurate.
We have optimized the geometries and calculated the carbon NMR for various heterocyclic moleculesfor understanding which members of rings are more stable Figs1-8.
Our methods and physical chemistry approach have been done based on our previous works19-44.
Results and Discussion
Optimization& NMR constants with orientations of the principal components&Haeberlen-Mehring17, orHerzfeld-Berger16-18 parameterfor several heterocyclic compounds inrandom situations has been calculated through DFT methods tables1,2.
In small distance around the center, the asymmetric-parameter16(η), and the skew17(κ), exhibited.gaussian distribution based on their fluctuation behavior15, which is relate on its distanceof molecular ring. In contrast, of that parameters, the isotropy16-18 Has not a fluctuating behavior and increase in aroundthe center of the rings with a linear relationship15. The slopes of that line is changedfor various distances ofheterocyclic compounds.
The isotropy16-18in allNMRcalculations are positive which indicates negative values for aromaticity15.
It is obvious that the isotropies15for NICS data can explain the quantity and quality of the aromaticity for some molecules, but those are not able for expressing the mechanism as well as S-NICS15.
In the S-NICS method15 the suitable shielding space near to the center of hetero ringsare enables to evaluate the aromaticity15as a criterion data. and in this method the expectation of the (η*)16-18 and (κ*)16-18have been estimatedvia the Gaussian curve.
The isotropy(σiso*) which is related to all of (η*),(κ *),(Ω*) and ( *) is suitable criterion for the aromatic molecules both hetero or regular ringsthrough the S-NICS method. Similar to the NICS method, in S-NICS15, mines nucleus-independent-chemical-shifts indicates thearomaticity.Therefor “+” values indicatethe anti-aromaticity quantitative. In S-NICS15 methods, the shielding& de-shielding15 spaces are importantfor discussing the mechanism of the aromatic molecules in point of ring currents.
The stabilitiesS-NICScriterion is stronglyaffected on the best places in shielding spaces which is relatedto the composition of hetero aromatic rings. It is obvious that geometry factors cause changes in the magnetic-field15 by the nuclei and change the resonant frequencies. Therefore the chemical shielding and several factors as the same electronegativity, magnetic anisotropy of π-systems will be changed due to the number of electrons The chemical shielding is a vector orientation function for all of the shielding parameters that can change in several places inside the shielding region.
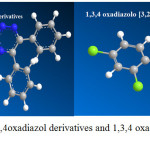 |
Figure 1: optimization of 1,3,4oxadiazol derivatives and 1,3,4 oxadiazolo [3,2,] 1,3,5 triazine 5 thione |
![Figure 2: optimization of 1,3,4 oxadiazolo,3,2,s triazin 7 thione and 3 subtstituted-pyrido[3,4]as-triazines(2).](http://www.orientjchem.org/wp-content/uploads/2017/07/Vol33No4_NIC_Nay_fig21-150x150.jpg) |
Figure 2: optimization of 1,3,4 oxadiazolo,3,2,s triazin 7 thione and 3 subtstituted-pyrido[3,4]as-triazines(2). |
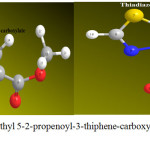 |
Figure 3: optimization of Methyl 5-2-propenoyl-3-thiphene-carboxylate and Thiadiazolo-pyrimidines derivatives (1)
|
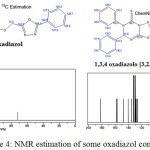 |
Figure 4: NMR estimation of some oxadiazol compounds |
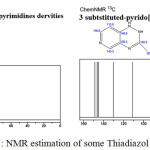 |
Figure 5: NMR estimation of some Thiadiazol compounds |
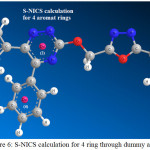 |
Figure 6: S-NICS calculation for 4 ring through dummy atoms
|
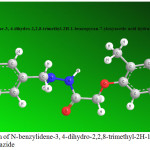 |
Figure 7: optimization of N-benzylidene-3, 4-dihydro-2,2,8-trimethyl-2H-1-benzopyran-7-yloxyacetie acid hydrazide |
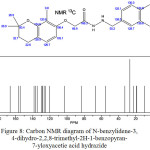 |
Figure 8: Carbon NMR diagram of N-benzylidene-3, 4-dihydro-2,2,8-trimethyl-2H-1-benzopyran-7-yloxyacetie acid hydrazide |
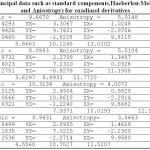 |
Table 1: the principal data such as standard components, Haeberlen-Mehring (Isotropic and Anisotropy) for oxadiazol derivatives |
Table 2: S-NICS calculation for 14 center rings
| Dummy atoms in Rings(number) | σ11 | σ22 | σ33 | S-NICS | NICS |
| BQ1 | 5.8663 | 10.1245 | 13.0102 | -9.667 | -9.122 |
| BQ2 | 3.6297 | 8.8933 | 11.7720 | -8.098 | -9.051 |
| BQ3 | 7.9371 | 10.0193 | 12.9854 | -10.313 | -9.265 |
| BQ4 | 4.5560 | 10.7527 | 11.5207 | -8.943 | -9.034 |
| BQ5 | 4.9321 | 9.3453 | 11.3456 | -8.541 | -9.01 |
| BQ6 | 5.0031 | 9.1267 | 10.9934 | -8.374 | -8.756 |
| BQ7 | 4.9835 | 9.3476 | 11.4523 | -8.594 | -8.765 |
| BQ8 | 5.1211 | 9.8745 | 10.9874 | -8.661 | -9.001 |
| BQ9 | 4.9135 | 11.84312 | 10.8734 | -9.211 | -9.324 |
| BQ10 | 6.6345 | 10.9833 | 11.9823 | -9.866 | -9.545 |
| BQ11 | 7.3462 | 11.0987 | 10.9872 | -9.811 | -9.934 |
| BQ12 | 4.9932 | 10.9453 | 11.9352 | -9.291 | -9.012 |
| BQ13 | 5.0923 | 10.9542 | 10.9987 | -9.015 | -9.241 |
| BQ14 | 6.9345 | 9.4581 | 11.0187 | -9.137 | -9.345 |
Refrences
- Gomtsyan, A. Chem. Heterocycl. Compd. 2012, 48,7–10.
CrossRef - Dua, R.; Shrivastava, S.; Sonwane, S.K.; Srivastava, S.K. Adv. Biol. Res. (Rennes). 2011, 5, 120–144.
- Broughton, H.B.; Watson, I.A. J. Mol. Graph. Model.2004, 23, 51–58.
CrossRef - El-salam, N.M.A.; Mostafa, M.S.; Ahmed, G.A.; Alothman, O.Y. J. Chem. 2013, 2013, 1–8.
- Azab, M.E.; Youssef, M.M.; El-Bordany, E.A. Molecules 2013, 18, 832–844.
CrossRef - Salem, M.S.; Sakr, S.I.; El-Senousy, W.M.; Madkour, H.M.F. Arch. Pharm. (Weinheim). 2013, 346, 766–773.
CrossRef - Cao, X.; Sun, Z.; Cao, Y.; Wang, R.; Cai, T.; Chu, W.; Hu, W.; Yang, Y. J. Med. Chem. 2014, 57, 3687–3706.
CrossRef - El-Sawy, E.R.; Ebaid, M.S.; Abo-Salem, H.M.; Al-Sehemi, A.G.; Mandour, A.H. Arab. J. Chem. 2013, 7, 914–923.
CrossRef - Chen, Y.; Yu, K.; Tan, N.Y.; Qiu, R.H.; Liu, W.; Luo, N.L.; Tong, L.; Au, C.T.; Luo, Z.Q.; Yin, S.F.Eur. J. Med. Chem. 2014, 79, 391–398.
CrossRef - El-Sawy, E.R.; Mandour, A.H.; El-Hallouty, S.M.; Shaker, K.H.; Abo-Salem, H.M. Arab. J. Chem. 2013, 6, 67–78.
CrossRef - Mabkhot, Y.N.; Barakat, A.; Al-Majid, A.M.; Alshahrani, S.; Yousuf, S.; Choudhary, M.I. Chem. Cent. J. 2013, 7, 112–120.
CrossRef - Vitaku, E.; Smith, D.T.; Njardarson, J.T. J. Med. Chem. 2014, 57, 10257–10274.
CrossRef - Verma, A.; Joshi, S.; Singh, D. J. Chem. 2013, 1–12]
- D. S. Arora, Singh, J. and Aneja, K. R.Kluwer Academic/Plenum Publishers, New Yok,1999
- Monajjemi, M.; Mohammadian, T Nayyer. J. Comput. Theor.Nanosci.2015,12, 4895-4914
CrossRef - J. Herzfeld, A. E. Berger, J. Chem. Phys.1980, 73, 6021
CrossRef - U. Haeberlen, In Advances in Magnetic Resonance, Suppl. 1 Academic Press, New York (1976), M. Mehring.M, Principles of High Resolution NMR in Solids, 2nd.ed, Springer Verlag, Berlin, H. W. Spiess, In NMR Basic Principles and Progress; P. Diehl, E. Fluck, R. Kosfeld, Eds.; Springer Verlag, Berlin, 1978,15.
- D. P, Raleigh, F. Creuzet, S. K Das Gupta, M. H Levitt, and R. G Griffin. J. Am. Chem. Soc.1989,111, 4502
- Monajjemi, M.; Lee, V.S.; Khaleghian, M.; B. Honarparvar, B.; F. Mollaamin, F. J. Phys.Chem C. 2010, 114, 15315
CrossRef - Monajjemi, M, Journal of Molecular Liquids, 2017, 230 , 461–472
CrossRef - Jalilian,H.; Monajjemi, M. Japanese Journal of Applied Physics. 2015, 54(8), 08510
CrossRef - Monajjemi, M.Struct Chem.2012, 23,551–580
CrossRef - Monajjemi, M.; Boggs, J.E. J. Phys. Chem. A, 2013,117,1670 −1684
CrossRef - Mollaamin, F.; Monajjemi, M, Journal of Computational and Theoretical Nanoscience. 2012, 9 (4) 597-601
CrossRef - Monajjemi, M.; Khaleghian, M, Journal of Cluster Science. 2011, 22(4),673-692318
CrossRef - Monajjemi, M.; Wayne Jr, Robert. Boggs, J.E. Chemical Physics.2014, 433, 1-11
- Monajjemi, M. Falahati, M.; Mollaamin, F.; Ionics, 2013, 19, 155–164
CrossRef - Monajjemi, M.; Mollaamin, F. Journal of Cluster Science, 2012, 23(2), 259-272
CrossRef - Tahan, A.; Monajjemi, M. Acta Biotheor,2011, 59, 291–312
CrossRef - Mollaamin, F.; Monajjemi, M.Physics and Chemistry of Liquids .2012, 50(5) ,596–604
CrossRef - Monajjemi, M.; Khosravi, M.; Honarparvar, B.; Mollaamin, F.; International Journal of Quantum Chemistry, 2011, 111, 2771–2777
CrossRef - Monajjemi, M. TheorChemAcc, 134:77 DOI 10.1007/s00214-015-1668-9,2015.
- Monajjemi, M. Journal of Molecular Modeling, 2014, 20, 2507
CrossRef - Monajjemi, M.; Khaleghian, M.; Mollaamin, F. Molecular Simulation. 2010, 36(11), 865–
CrossRef - Monajjemi, M. Biophysical Chemistry.2015, 207,114 –127
CrossRef - Monajjemi, M.; Honaparvar, B.; Hadad, B. Khalili, African Journal of Pharmacy and Pharmacology , 2010, 4( 8),521-529
- Mollaamin, F.; Varmaghani, Z.; Monajjemi, M. Physics and Chemistry of Liquids 2011,49( 3) 318-336
CrossRef - Monajjemi, M.; Rajaeian, E.; Mollaamin, F, Physics and Chemistry of Liquids 2008,46( 3 )299-306
CrossRef - Monajjemi, M.; Heshmat, M.; Aghaei, H.; Bulletin of the Chemical Society of Ethiopia ,21( 1) 111-116
- Monajjemi, M.; Aghaie, H.; Naderi, F. Biochemistry (Moscow) 2007, 72 ( 6 ),652-657
CrossRef - Monajjemi, M.; Honarparvar, B.; Nasseri, S. M. Journal of Structural Chemistry 2009, 50( 1 )67-77
CrossRef - Mollaamin, F.; Baei, M. T.; Monajjemi, M.; Russian Journal of Physical Chemistry A, 2008, 82 (13),2354-2361
- Monajjemi, M.; Baei, M. T.; Mollaamin, F. Russian Journal of Inorganic Chemistry ,2008, 53 ( 9 ),1430-1437
CrossRef - Monajemi, M.; Ketabi, S.; Zadeh, MH; Biochemistry-Moscow 2006, 71, S1-S8
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









