Preparation and Study of Catalysts on Metal Blocks for Neutralization of Exhaust Gases of the Stationary Diesel Generator
Larissa Sassykova1, Arailym Nalibayeva2, Yermek Aubakirov1, Zheneta Tashmukhambetova1, Ulzhan Otzhan1, Nurbubi Zhakirova1 and Maria Faizullaeva3
1Al-Farabi Kazakh National University, 71, al-Farabi ave., 050040, Almaty, Kazakhstan.
2D.V.Sokolsky Institute of Fuel, Catalysis and Electrochemistry, 142, D.Kunaev str., 050010, Almaty, Kazakhstan.
3Scientific Research Institute for New Chemical Technologies and Materials, 95a, Karasaibatyr str., 050012, Almaty, Kazakhstan.
Corresponding Author E-mail: larissa.rav@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/330440
The work aim was preparation of catalysts with various active phase on metal block carriers and test their effectiveness on exhaust gases of the stationary diesel generator at various loadings. The active phase was prepared on the basis of the platinum group metals, oxides of nickel, manganese. The catalyst on the basis of 0.1% of Pt kept the activity during 100 h. test on the diesel generator. The most effective catalyst at all engine operating conditions was the sample containing 5% of nickel oxide and manganese and activated of 0.1% Pt. The catalytic samples on the basis of Ni and Mn promoted by Pd (0.1%, 0.25%) and Pt (0.1%), provide high degree of conversion of CO to CO2, СхНy to CO2 and H2O, NO to N2.
KEYWORDS:Exhaust gases; catalysts on the metal blocks; neutralization of emissions; diesel generator
Download this article as:| Copy the following to cite this article: Sassykova L, Nalibayeva A, Aubakirov Y, Tashmukhambetova Z, Otzhan U, Zhakirova N, Faizullaeva M. Preparation and Study of Catalysts on Metal Blocks for Neutralization of Exhaust Gases of the Stationary Diesel Generator. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Sassykova L, Nalibayeva A, Aubakirov Y, Tashmukhambetova Z, Otzhan U, Zhakirova N, Faizullaeva M. Preparation and Study of Catalysts on Metal Blocks for Neutralization of Exhaust Gases of the Stationary Diesel Generator. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35277 |
Introduction
Reducing air pollution by toxic substances emitted by industrial plants and motor vehicles is one of the most urgent tasks for all industrialized and economically developed countries. A crucial role in air pollution is being played gasoline internal combustion engines. However, the reduction in toxicity of diesel vehicles also deserves serious attention. Diesel engines and diesel generators were widely adopted in all spheres of transport and the industry. Thanks to diesel engines more high level of effectiveness of use of fuel in comparison with gasoline engines is reached: the modern diesel units spend fuels for about 30.0% less, than gasoline motors with direct injection of the same generation1-5. Nevertheless, at operation of diesel generators arise the dangerous and harmful factors (noise, vibration, exhaust gases) as a result of which action the ecological situation is broken, the exhaust gases of diesel engines contain significantly higher concentration of solid particles which existence in air can be the cause of some serious illness, including oncological violations, and even premature death6-8. It is very important issue of cleaning of exhaust gases in closed spaces where are operated the stationary diesel generators in limited spaces: in the mines, ports, quarries of mines of the opencast mining. Diesel engines are a major source of nitrogen oxides in the atmosphere which causes harmful effects on human health, resulting in the formation of ozone and particulate matters containing carcinogenic substances. The main directions of improvement of diesel engines are reducing of fuel consumption, toxicity of the exhaust emissions, noise level, engine capacity increase, facilitation of cold start. The composition of the exhaust gases of gasoline and diesel is significantly varied. For the engines working at gasoline is characteristic lower content of oxygen, high – of carbon dioxide and especially large – of carbon monoxide.
Among the known methods of disposal and neutralization of harmful emissions of transport the most effective is the deep catalytic oxidation of organic compounds to carbon dioxide and water. The catalysts on block metal or ceramic carriers are the most preferable thanks to the developed surface, a wide choice of options of chemical and design decisions, low difference of pressure, high thermal and mechanical stability. Effective use of metal in the active phase when preparing of catalysts on metal carriers is achieved in case if the active phase is transferred into the highly dispersed condition. Protection of the environment from industrial and transport pollution puts to humanity demands to improve the synthesis methods of the neutralizing catalysts and purification of gas emissions from harmful admixtures9-12. The urgency of creating a reliable working neutralizers increases due to necessity of compliance with current environmental standards8, 13.
The work aim is preparation of full-size catalysts on metal block carriers and test of their effectiveness on exhaust gases of the stationary diesel generator at various loadings.
Materials and Methods
By the technique developed earlier14-16 the catalysts on metal block carriers (fig.1) are synthesized.
For preparation of metallic carriers for full size samples of catalysts was used the heat-resistant foil of 50 microns thick with gauge length and width, which is goffered (fig.1). It applies two types of corrugation. The first type of the corrugations (1a) has the usual form channels, while the 2nd type of the corrugated foil (fig. 1 b) is curved at the inlet gas stream in the middle and at the outlet. This form of channels promotes destruction of the laminar gas flow, the formation of turbulence and increases the degree of contact of the unreacted gas molecules with an active catalyst layer. The total length of one channel of foil of the second type (fig.1, b) sample by 5.0% longer than of the sample of 1-st type (fig.1,a). The foil is located in vertical position on installation for goffering, goffering is carried out with the help of platens with diameter of 50.0 mm and length 250.0 mm. The tape is kept in the centre of platens with the help of directing cheeks. On the smooth tape foils is superimposed a corrugated tape, then the strips are folded in the cylinder block and joined by welding contact.
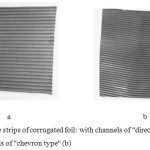 |
Figure 1: The strips of corrugated foil: with channels of “direct” type (a), with channels of “chevron type” (b) |
Fig.2 shows block metal carriers with honeycomb structure of channels. The washcoat (secondary carrier) is put on the prepared metal carrier. The washcoat was prepared on the basis of alumina or alumina with additives (compounds of refractory metals or compositions on the basis of zeolites).
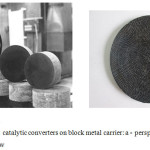 |
Figure 2: The full-size catalytic converters on block metal carrier: a – perspective view, b – cross-sectional view |
During synthesis of catalysts viscosity of suspension on the basis of alumina was defined, its best value for suspension preparation was equal to – 0.02 Pa·sec. Removal surplus of suspension from channels of the block carrier is carried out with use of the Centrifuge CM-6M. It was found experimentally that for the removal of the excess slurry of washcoat from the channels block is required speed of rotation of the centrifuge 600 rev/min., duration rotation-2.0 min. After drying blocks in a furnace the active phase of the catalyst is deposited on the washcoat. The active phase is prepared on the basis of platinum metals, oxides of nickel and manganese by dissolution of compounds of the corresponding metals (nitrates, acetates, formates) in decationized water. Also polyethylene – glycol – PEG-10, 000 for synthesis of the active phase is used. The active phase is deposited on the metal blocks covered with the washcoat by means of impregnation. After that drying of the impregnated blocks is carried out at a temperature of 473 K during 4 h. and calcination of samples at 773 K during 2 h. Some of samples of catalysts was promoted with an aqueous solution of PdCl2, of 0.2% by weight, the other part of catalysts was promoted by Pt, of 0.1% by weight.
For testing the efficiency of the catalysts was used the stand on the basis of diesel generator of brand 5GF-LDE with power of 5 kVA (fig.3, 4). The full-size catalyst samples had a diameter of 30.0 mm, a height of 90.0 mm. The catalyst was loaded into the gas-abstersive offshoot of the diesel generator. Temperature before and after the catalyst is defined with use of a chromel-alumel thermocouple outputted on the digital indicator. Gas mixture was analyzed by means of GLC and on a gas analyzer “OPTOGAS 500” before and after the reaction. The chromatographs “Crystal 2000M” and “Chrom 3700” with a flame ionization detector were used. Analysis duration was equal to 20-30 min. Previously the catalyst was calcined at 773 K for 4 h. in air in a muffle furnace.
 |
Figure 3: The catalytic unit (the stand) on the basis of diesel generator Click here to View figure |
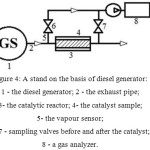 |
Figure 4: Principle schema of the stand on the basis of diesel generator: 1 – the diesel generator; 2 – the exhaust pipe; 3- the catalytic reactor; 4- the catalyst sample; 5- the vapour sensor; 6, 7 – sampling valves before and after the catalyst; 8 – a gas analyzer. Click here to View figure |
Results and Discussion
Tests were carried out on all modes of operation of the engine of the diesel generator (at no-load operation (idling), at 1.0; 2.0; 3.0; 4.0 kVA). The results of triple tests of catalysts are presented in Table 1. With an increasing number of engine revolutions per minute from 420.0 to 820.0 temperature rise is observed from 491 to 851 K and increase of carbon monoxide content from 0.08 to 0.26%. The content of hydrocarbons at the same time was varied within 0.002-0.008,4 vol.%. The analysis of exhaust gases before and after the catalyst showed that the lowest percentage of oxidation of harmful components was characteristic of modes of engine power – up to 5.0 kVA, revolutions per min. – to 490.0, when the gases have a low temperature (up to 553 K). With increasing of engine load the degree of deep oxidation of harmful components increased and stored in the area of CO values – from 40.0 to 90.0%, hydrocarbons from 35.0 to 100% for nitrogen oxides from 10.0 to 40.0 %. Thus, the synthesized catalysts at 473 K and higher provide a high performance in stand tests.
Bench tests of three catalysts (on the basis of Ni-Mn promoted by Pd (0.1-0.2 wt.%) and Pt (0.1 wt.%) are carried out.
Table 1: The effectiveness of the complex cleaning of exhaust gases on the full-size block catalysts on the basis of 0.1 and 0.25% Pd, 0.1% Pt
| Number of rev/ min.(at different power, kVA) |
Counter pressure, mm of a hydrogen column
|
Temperature, K |
CO, ppm |
СхНy, ppm |
NOx, ppm |
||||||
| before neutra-lizer | afterneutra-lizer | %clea-ning | before neutralizer | afterneutralizer | %cleaning | before neutralizer | afterneutralizer | %cleaning | |||
|
460 (1) |
110 |
491 |
0.085 |
0.085 |
0.0 |
0.0
|
0.0
|
0.0
|
0.072
|
0.05
|
30.6
|
|
490 (2) |
128 |
553 |
0.15 |
0.047 |
69.7 |
0.48
|
0.02
|
58.4
|
0.026
|
0.02
|
23.1
|
|
570 (3) |
144 |
673 |
0.145 |
0.09 |
38.0 |
0.49
|
0.02
|
59.3
|
0.072
|
0.05
|
30.6
|
|
660 (4) |
156 |
573 |
0.152 |
0.052 |
65.8 |
0.48
|
0.02
|
58.4
|
0.068
|
0.03
|
55.9
|
|
750 (5) |
171 |
843 |
0.150 |
0.02 |
78.7 |
0.20 |
0.0 |
100 |
0.064 |
0.026 |
61.8
|
|
420 (1) |
94 |
491 |
0.080
|
0.05
|
37.5
|
0.37
|
0.02
|
334
|
0.08
|
0.05
|
37.5
|
|
490 (2) |
120 |
553 |
0.125
|
0.085
|
32.0
|
0.37
|
0.02
|
409
|
0.05 |
0.02 |
69.0 |
|
580 (3) |
134 |
673 |
0.157
|
0.045
|
71.4
|
0.36
|
0.03
|
259
|
0.09
|
0.03
|
68.7
|
|
660 (4) |
155 |
573 |
0.165
|
0.060
|
64.0
|
0.34
|
0.04
|
100
|
0.05
|
0.04
|
20.0
|
|
740 (5) |
174 |
843 |
0.215 |
0.080 |
63.0 |
0.44 |
0.02 |
100 |
0.05 |
0.03 |
51.8
|
|
420 (1) |
81 |
493 |
0.083
|
0.072
|
13.2
|
0.67
|
0.40
|
40.5
|
0.08
|
0.06
|
27.5
|
|
485 (2) |
121 |
551 |
0.072
|
0.475
|
34.5
|
0.37
|
0.32
|
14.0
|
0.04
|
0.03
|
19.1
|
|
580 (3) |
128 |
578 |
0.137
|
0.067
|
51.3
|
0.42
|
0.27
|
35.4
|
0.09
|
0.04
|
58.4
|
|
660 (4) |
147 |
493 |
0.152
|
0.110
|
27.9
|
0.24
|
0.20
|
92.0
|
0.10
|
0.05
|
56.6
|
|
750 (5) |
164 |
578 |
0.212 |
0.021 |
100 |
0.64 |
0.06 |
100 |
0.06 |
0.002 |
100
|
Tables 2, 3 show the test data. It is found that in CO oxidizing reaction the catalyst containing 0.2% of Pd is more active, than the catalyst on the basis of Mn and Ni oxides. Under identical test conditions in reactions of reduction of nitrogen oxides and oxidations of hydrocarbons activity of catalysts on the basis of Pd and Mn and Ni oxides is almost identical. High selectivity on NOx was observed at higher loadings of the engine (3.0-4.0 kVA) where temperature reached 600-700 K.
Table 2: Results of the analysis of toxiferous emissions on the diesel generator with use of 3.0% catalyst on the basis of Mn and Ni oxides
| Power consumption, kVA | The temperature of exhaust gases, K | The content of toxic components in the exhaust gas, ppm | |||||
|
CO |
СхНy |
NOx |
|||||
| Before catalyst | Aftercatalyst | Before catalyst | Aftercatalyst | Before catalyst | Aftercatalyst | ||
| Idling (0) |
298 |
0.036 | 0.035 | 65.0 | 60.0 | 13.2 | 13.2 |
|
2.0 |
533 |
0.030 | 0.00 | 81.0 | 20.0 | 17.4 | 10.8 |
|
3.0 |
573 |
0.021 | 0.00 | 89.0 | 3.5 | 19.0 | 7.6 |
|
4.0 |
698 |
0.014 | 0.00 | 111.0 | 10.0 | 23.0 | 8.0 |
Table 3: Results of the analysis of toxic emissions of diesel generator by using a catalyst containing 0.2% Pd
| Power consum-ption, kVA | Tempera-ture of exhaust gases, K | The content of toxic components in the exhaust gas, ppm | |||||
|
CO |
СхНy |
NOx |
|||||
| Before catalyst | Aftercatalyst | Before catalyst | Aftercatalyst | Before catalyst | Aftercatalyst | ||
| Idling (0) |
298 |
0.033 |
0.033 |
67.2 |
55.1 |
14.5 |
14.1 |
|
2.0 |
533 |
0,03 |
0.002 |
79.6 |
62.3 |
17.8 |
8.3 |
|
3.0 |
573 |
0.02 |
0.00 |
92.1 |
6.4 |
21.0 |
6.4 |
|
4.0 |
698 |
0.015 |
0.00 |
107.0 |
4.2 |
22.6 |
7.0 |
Catalysts based on palladium and platinum with a promoting additives were tested for thermal stability under load of diesel generator 3.0 kVA. During the 100-h. test with fractional neutralizers calcination at 873 K at an interval of 5 h. in a muffle furnace it was found that the introduced structural and textural thermo stabilizing additives into the catalysts promote a sustainable activity (tab.4).
Table 4: Investigation of thermal stability Pt- and Pd-containing catalysts on diesel generator
| The catalyst | Exhaust gases | Initial degree of cleaning, % |
Duration of testing, h. |
||||
|
5 |
10 |
25 |
50 |
100 |
|||
| 0.1% Pt+Me |
CO |
100 |
100 |
100 |
100 |
100 |
100 |
|
СхНy |
95.0 |
95.3 |
94.2 |
94.3 |
94.2 |
94.7 |
|
|
NOx |
60.5 |
60.0 |
61.0 |
59.8 |
59.7 |
59.6 |
|
| 0.2% Pd+Me |
CO |
100 |
100 |
100 |
100 |
100 |
100 |
|
СхНy |
90.0 |
90.2 |
90.0 |
88.7 |
88.8 |
88.4 |
|
|
NOx |
48.2 |
42.9 |
48.1 |
48.1 |
46.8 |
46.4 |
|
Diesel exhaust is a mixture of coarsely dispersed aerosol with a particle size of 0.5-1.0 microns14, 17-19. The disperse phase of the same mixed aerosol has complex composition and consists of liquid and resinous and solid unburned products. Experiments carried out on the diesel generator in this investigation showed that in the diesel exhaust at all engine operation modes is formed soot with the with a predominant size of particles 0.4-5.0 micrometres. The speed of these particles reached 20 m/s, and the temperature – 673 K. Primary structures of the soot which is formed in diesel combustion gases consists – are the particles of spherical shape with a diameter of 150 — 1,700 Å with a specific surface area to 70.0-76.0 м2/g. Already in the course of combustion there is a coagulation of soot particles and formation of secondary and tertiary structures. Soot in the fulfilled gases of diesels represents formations of irregular shape. In the process of expansion of gases in the diesel cylinder to soot particles oxygen enters (due to gas movement and diffusion of oxygen), i.e. the favorable conditions for a soot burnup are created. The researches of formation and burnup of soot in the cylinder of the diesel engine executed by method of a spectral analysis20-22 show that the considerable proportion of soot burns out in the course of expansion. Isolation of soot from the exhaust gases of diesel engines depends, probably from both the process of formation and the process of its burning. Formation of soot can happen in places where there is excess fuel or at hit of streams of fuel on rather cold walls of the cylinder. The solid particles of soot moving along the channels of the catalyst used in the tests function as an abrasive material on the catalyst surface, thereby partly carrying away an active phase of catalyst. On the other hand, part of them is adsorbed on the surface of the catalyst, reducing its working surface. Nevertheless, the studied Pt- and Pd-containing catalysts in these conditions within 1 month practically did not lose their effectiveness in oxidizing reactions of CO, hydrocarbons and reduction of nitrogen oxides. This indicates a high adhesion strength and chemical resistance to the products of diesel engine exhaust.
Tests of catalysts for the purpose of determination of stability of catalysts to the poisons containing in exhaust gases of the diesel engine are carried out. During research of three types of catalysts it was found that Pt-containing catalyst (0.1% of Pt) on the basis of alumina at a temperature of exhaust gases 573 K oxidizes for 100% CO and for 92.0% hydrocarbons and partially to 17.0% transform nitrogen oxides. Further increase of loading of the engine does not influence a rate of oxidation of CO and hydrocarbons. Similarly also Pd – containing (0.2%) catalyst on alumina behaves, oxidizing CO for 100% and СхНy for 90.0% at 573 K. At the same time, the Pt catalyst prepared on the basis of alumina and 10.0% zeolite HY, showed significantly higher activity for neutralizing of toxic diesel engine emissions. Thus, at 573 K the level of toxic emissions decreasing on CO reaches 100%, on СхНy– 100%, on NOx – 48.0%. A further increase in the load contributes to lower content of NOx to 22.0%, without reducing of the level of purification for CO and СхНy.
By method of the X-ray phase analysis and EM14 the physical and chemical researches of catalysts were carried out. It was found that the oxide catalysts represent spinels with a cubic lattice with NiMnO4 peaks 2Å, 52Å, 148Å, 203Å. There are also low-intensity peaks of alumina (160Å, 256Å), in the catalysts based manganese on are formed the particles which are finely dispersed, uniformly distributed on the surface of the carrier. X-ray analysis showed Pd and Pt scattering spectrum, which indicates a high dispersion of the metal.
In fig. 5 the nano-dimensional particles of Pt and Pd obtained at synthesis of the active phase of catalysts by introduction of the corresponding salts to an aqueous solution of PEG are shown. Pictures are made with use of electron microscope EM-125 K. Pt particle sizes are 7-8 nm, Pd-11 nm.
 |
Figure 5: The nanosized particles (300, 000 magnification): Pt (a), Pd (b) |
By use of a method of TPD of ammonia the acid properties of the prepared platinum and palladium catalysts were studied14. The total concentration of acid centers Pt- and Pd-promoted catalysts of nickel and manganese oxides, higher than in the initial alumina-platinum and palladium catalysts. Thus, Pt-and Pd-containing catalysts at close values of the total concentration of the acid centres 660.0-620.0 μmol/g are characterized by a distinguished ratio of the centres of different force, that, apparently, determines distinctions in their catalytic properties. In fig.6 the comparative diagrams for catalysts with various concentrations of the acid centres of different force were shown. Catalysts on basis Pt/Ni-Mn/Al2O3 are characterized by the greatest concentration of the strong acid centres (280.0 μmol/g), for Pd/Ni-Mn/Al2O3 catalyst the greatest concentration of the weak acid centres – 250.0 μmol/g.
It was found, that in manganese catalysts on basis PEG were formed the particles which were finely divided, in regular intervals distributed on a surface of the carrier and that fact was proved to be true as well results of XRD analysis. At transition to the catalysts on the basis of acetate, and, in particular, to catalysts on the basis of nitrates of manganese, there was an integration of particles that was, apparently, the reason of decrease in activity of manganese catalysts in reaction of oxidation of hydrocarbons and CO.
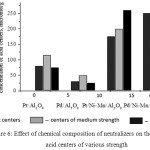 |
Figure 6: Effect of chemical composition of neutralizers on the distribution acid centers of various strength |
Conclusion
The obtained results of studying of activity of catalysts with various active phase on metal block carriers on exhaust gases of the stationary diesel generator at various loadings show that the prepared catalysts at 473 K and above provide high performance in bench tests. The catalyst on base 0.1% of Pt during bench tests worked on the diesel generator more than 100 h. without decrease of efficiency. At all engine operating conditions the most effective sample was catalyst containing 5% of oxides of nickel and manganese, activated by 0.1% of Pt. The catalytic samples on the basis of Ni and Mn promoted by Pd (0.1%, 0.25%) and Pt (0.1%), provide high degree of transformation CO to CO2, СхНy into CO2 and H2O, NO to N2.
References
- Mathieu, O.; Lavy J.; Jeudy E. J.Topics in Catalysis 2009, 52(13-20), 1893-1897
- O’Neill, B.C. J.Science 2002, 5575, 1971.
CrossRef - Rauch, S.; Harold, H.F.; Barbante, C.; Masanori, O.; Morrison, G.M.; Peucker-Ehrenbrink, B.; Wass, U. J.Environ.Sci.Technol. 2005, 1, 8156
CrossRef - Takami, A.; Ichikawa, T. J. Zeolites. 1995, 15(3), 283
- Val’dberg, A.Yu; Kosogorova, T.O.; Tsedilin, A.N.; Pokrovskii, D.D.; Yakimychev, A.A.; J.Chemical and Petroleum Engineering. 2007, 5-6, 287-291
- Awofeso, N. American Journal of Respiratory and Critical Care Medicine. 2011, 10, 1437
CrossRef - Giraldo, L.; Moreno-Pirajan, J.C. Orient. J. Chem. 2014, 30(2), 451-461
CrossRef - Kołodziej, A.; Łojewska, J. Chapter in: New and Future Developments in Catalysis. 2013, 257-279
- Tsai, J.-H. J.Aerosol and Air quality research. 2015. http://dx.doi.org/10.4209/aaqr.2015.07.0482.
CrossRef - Kramer, M.; Schmidt, T.; Stowe, K.; Maier, W.F. J.Appl.Catalysis A: General. 2006, 302, 257–263
CrossRef - Yang, S.; He, L-Y. J.Energy & Environment. 2016. http://dx.doi.org/10.1177/0958305×15627545.
CrossRef - McGrath, M. Four major cities move to ban diesel vehicles by 2025. http://www.bbc.com/news/science-environment-38170794.
- Sendilvelan, S.; Bhaskar, K. Rasayan J.Chem. 2016, 9(4), 692-696
- Sassykova, L.R.; Massenova, A.T.; Gilmundinov, Sh.A.; Bunin, V.N.; Rakhmetova, K.S. DGMK, Tagungsbericht, 2014, 3, 181-188
- Sassykova, L.; Gil’mundinov, Sh.; Nalibayeva, A.; Bogdanova, I. Rev. Roum. Chim. 2017, 62(2), 107-114
- Sassykova, L.R.; Ussenov A.; Massenova, A.T.; Gil’mundinov, Sh.A.; Rakhmetova, K.S.; Bunin, V.N.; Basheva, Zh.T.; Kalykberdiyev, M.K. Int. J. Chem. Sci. 2016, 14(1), 206-212
- Burdeinaya, T.N.; Matyshak, V.A.; Tret’yakov, V.F.; Glebov, L.S.; Zakirova, A.G.; Carvajal, G., Villanueva, A.M.E. J.Appl.Catalysis B:Environmental. 2007, 1-4, 128.
CrossRef - Lee, B.Y.; Inoue, Y.; Yasimori, I. J Bull Chem Soc Jpn. 1981, 54, 3711.
CrossRef - In, B.D.; Kim, H.; Son, J.; Lee, K. Intern. J. Heat and Mass Transf. 2015, 86, 667 – 680.
CrossRef - Nalibayeva, A.; Sassykova, L.R.; Kotova, G.N.; Bogdanova, I.O. News of National Academy of RK, series of Chemistry and Technology. 2016, 419(5), 55-64
- Sassykova, L.R.; Nalibayeva, A.; Gil’mundinov, Sh.A. Bulgarian Chemical Comm. 2017, 49(3)
- Baiseitov, D.A.; Tulepov, M.I.; Tursynbek, S.; Sassykova, L.R.; Nazhipkyzy, M.; Gabdrashova,Sh.E.; Kazakov,Y.V.; Pustovalov,I.O.; Abdrakova, F.Y.; Mansurov, Z.A.; Dalton, A.B. Rasayan J. Chem.2017, 10(2), 344 -348.

This work is licensed under a Creative Commons Attribution 4.0 International License.









