Formation Process of Interpenetration Polymer Networking Composite Base on Poly Composite Polyurethane-Natural Rubber Assisted Montmorillonite as A Filler
Tamrin1, Rikson Siburian1,2 and Barita Aritonang3,4
1Chemistry Department-Faculty of Mathematic and Natural Sciences, University of Sumatera Utara-Medan-Indonesia.
2Nanomedicine Center and Stem Cell Center, University of Sumatera Utara.
3Chemistry-Post Graduated, University of Sumatera Utara JL. Biotekhnologi, No.1 Medan, P.Bulan, Kampus USU (20155).
4Chemistry Department-Universitas Sari Mutiara, Medan.
Corresponding Author E-mail: Thamrinusu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330446
The formation process of InterpenetrationPolymer Network (IPN) base on poly blend polyurethane (PU)-SIR-5 Natural Rubber (NR) which assisted montmorillonite (MMT) as a filler was carried out.The PU was synthesized by mixingpolypropylene glycol and toluene diisocyanates (mole ratio 2: 1). NR was vulcanised by adding stearic Acid, zinc oxide, MBTS and sulfur, respectively. PU was mixed with NR at 140oC to generate IPN PU-NR. Then, IPN PU-NR- MMT composite may be produced by adding MMT. The IPN PU-NR-MMT composite was characterized by Tensile Strength Test, Water Absorption Test, SEM, FTIR and DSC, respectively. The results showed that a mixture of IPN composites increased with increasing MMT and its good elongation. Mechanical properties, physical and IPN composite morphology is influenced by the amount of MMT. It causesthere is a cross linking each other at the bond interface.
KEYWORDS:Interpenetrating polymer network (IPN); SIR-5 Natural Rubber (NR); Polyurethanes (PU); Montmorillonite (MMT)
Download this article as:| Copy the following to cite this article: Tamrin T, Rikson R, Aritonang B. Formation Process of Interpenetration Polymer Networking Composite Base on Poly Composite Polyurethane-Natural Rubber Assisted Montmorillonite as A Filler. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Tamrin T, Rikson R, Aritonang B. Formation Process of Interpenetration Polymer Networking Composite Base on Poly Composite Polyurethane-Natural Rubber Assisted Montmorillonite as A Filler. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35087 |
Introduction
Interpenetrating Polymer Network (IPN) was made by using cross linking material which was synthesized withcondensation, addisionand propagation reactionsas well as used several monomers.Then, monomer was polymerized and cross linked. IPN is made base on several polymers where it is difficult to well mixture and result two phase (heterogen phase).Therefore, many researchers solve this problem by using IPN. This way was expected to generate the better material properties than the mixing polymer method base on large temperature and production of polymer.
Vivek (2013)has reported the synthesize of IPN by using sequential method with trans-esterification reaction. It showed that the thermal stability of IPN material is better than the pure polystyrene. The properties of IPN which was produced by (polyurethane (50 % w/w): poly acrylic (50 % w/w) has a good mechanical and thermal properties but the hardness property is worst(Marinovic, 2010). Shoubing (2011)reported that IPN composite showed its thermal decomposition increase and may be used as well as waving mute. IPN may be produced base on composites of poly (3, 4-etylendiocsitiopene), Nitril Butadiene, andoxide polyetilene. The data showed the IPN has the high flexibility(Laurent, 2014). Nicolas (2014)also reported IPN was synthesized by mixing polyetilenoxide and Nitril Butadiene Rubber.Pseudo-IPN was a polymer networking where was synthesized from mixing2,4-toluenebiisocianat and 1,4-butanadiol. It showed the elastomeric property (Amrollahi, 2011). Therefore, synthesize of IPN is needed.
In this paper, we reported synthesize of IPN with mixing Natural Rubber (NR)and polyuretan under condition solvent of toluenebiisosianat (TBI) andglycol polypropylene. The IPN composite may assist with adding monmorillonit (MMT).Also the characterizations of IPN are reported (Tensile Strength Test, Water Absorption Test, SEM, FTIR and DSC, respectively). The important point of this paper we would like to clarify formation process of IPN composite base on polyurethanes, natural rubber and monmorillonit materials.
Material and Method
Synthesize IPN
Synthesize IPN was carried out by using several steps namely: i) Natural rubber (90 phr)which have been masticated (see in supporting information) was added stearic acid (2 phr). It was mixed in internal mixer at 40oC for 5minutes;ii) Adding 1 phr ZnO andmixedfor 1 menit; iii) Adding 1 phr MBTS andstirredfor5minutes;iv) Then, 0,5 phr sulphur was mixedand stirred for 1 hour; v) Subsequently, adding 10 phr polyurethaneand stirred for20minutes;vi) Finally, composite was compressed by using hot compressor as well as reference of ASTM D638 Tipe V.The molecule weight of natural rubber is shown in Table 1S (see in supporting information). The ratio amount of PU and NR are shown in Table 2S (see in supporting information).
Preparation of IPN Composite Withmontmorillonit as Filler
82 phr NR-PU was put in internal mixer and stirredat T = 140oC. Then, it was added 18 phr MMT and stirredfor 15 minutes.Finally, composite was compressed by using hot compressor as reference at ASTM D638 tipe V under conditionsT= 140oC for 15 minutes. Ratio amount of Pu : NR : MMT was shown in Table 3S (see in supporting information).
Characterization of IPN Composite
IPN composite was characterized by using tensile tester (see in supporting information), Scanning Electron Microscope(SEM), and Differential Scanning Calorimeter(DSC).
Result and Discussion
FTIR Data]
Hasil Analisa Gugus Fungsi Poliuretan dari Toluena Diisosianat dan Polipropilena Glikol
Formation of IPN composite on polyurethane was characterized by using FTIR data(Figure 1).
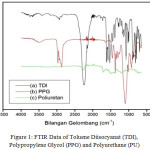 |
Figure 1: FTIR Data of Toluene Diisocyanat (TDI), Polypropylene Glycol (PPG) and Polyurethane (PU) |
FTIR data showadsorption band at 3299.92 cm-1, indicating gugus N-H functional group andwave number (1713,14 cm-1)appears as well as C=O (see supporting information Table 4S) . It is a character of urethanefrom polyurethane. In addition, the FTIRspectrum of polyurethane (2270.77 cm-1) is changed. It indicates polymerization occurs due to isocyanides group (N=C=O) decreases. The weak peak appears at 2270.7 cm-1, it means isocyanides still remains and reacts with polyol. Formation polyurethane is indicated by changing of functional group as well as reaction between TDI and PGG (see supporting information Table 5S and 6S), indicating polyurethane has good properties. Therefore, it may be used to synthesizeIPN composite.
Mechanic Properties
Mechanic tests (Universal System Mechine test) are carried out to understand well about effect of IPN with and without monmorillonit. Mechanic test data of IPN NR – PU (without montmorillonit) may be seen in Table 1.
Table 1: Mechanic test of IPN NR – PU
|
Ratio of NR : PU(phr) |
Stress(×10-3)(Mpa) |
Strain(%) |
Modulus of Elastisity(x10-3) (Mpa) |
|
100:0 |
12,56 |
65,13 |
19,28 |
|
90:10 |
22,22 |
82,02 |
27,09 |
|
80:20 |
23,11 |
96,21 |
24,02 |
|
70:30 |
15,71 |
86,84 |
18,09 |
|
60:40 |
10,68 |
37,24 |
28,68 |
|
50:50 |
0 |
0 |
0 |
|
40:60 |
0 |
0 |
0 |
|
30:70 |
0 |
0 |
0 |
|
20:80 |
– |
– |
– |
|
10:90 |
– |
– |
– |
|
0:100 |
49,25 |
5,53 |
890,60 |
Table 1 show the ratio of NR : PU (phr) 80 : 20 is the best among the others. The tensile strain, strength and modulus of elasticity increase with adding 20 phr polyurethane. In contrast, they will gradually decrease when PU amount is decreasing. It means amount of PU is very important to assist homogeny and compatible between NR and PU at IPN as well as to improve mechanic property. On the other hand, too much amount of PU will affect compatibility of NR and PU on IPN composite. It is consistent at ratio of PU (50, 60 and 70) where their tensile strength is zero. It means amount of PU is very important to produce IPN compatible and improving the mechanic properties of IPN composite. Furthermore, the stress and strain strength of ration NR: PU are shown in Figure 2 and 3, respectively.
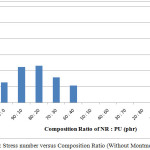 |
Figure 2: Stress number versus Composition Ratio (Without Montmorillonit) |
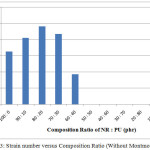 |
Figure 3: Strain number versus Composition Ratio (Without Montmorillonit) |
The stress tensile number of NR : PU composite is lower than the 100% of PU. It causes distribution of phase between NR and PU is weak thereby stress number of NR : PU composite will be go down. For strain number, NR : PU composite is higher than pristine NR (without PU). It is caused PU assists to increase strain tensile of NR due to PU contributes to increase interfacial bonding between NR and PU for IPN composite.
Then, montmorillonit effect for IPN composite beyond mechanic properties is shown in Table 2.
Table 2: Mechanic test of IPN Composite (With Montmorillonit)
|
Ratio of |
Stress(x10-3) |
Strain(%) |
Modulus of Elastisity |
|
NR-PU : MMT (phr) |
(Mpa) |
(10-3) (Mpa) |
|
|
82:18 |
6,48 |
65,78 |
9,85 |
|
80:20 |
9,98 |
92,97 |
10,73 |
|
78:22 |
10,67 |
109,89 |
9,71 |
|
76:24 |
12,31 |
124,58 |
9,88 |
|
74:26 |
18,92 |
150,14 |
12,60 |
|
72:28 |
14,13 |
140,00 |
10,09 |
|
70:30 |
6,92 |
107,29 |
6,45 |
|
68:32 |
6,00 |
96,94 |
6,19 |
The best composite base on mechanic properties is NR-PU : MMT (74 : 26). Stress and strain tensile of IPN composite with montmorillonit 26 phr as well as fillerincreasedue to interfacial bonding between NR-PU and MMU increase. It also may cause transition of pressure which generate good stress tensile. The other reasons, presence of montmorillonit contribute to homogenize of composite in term of synchronize phase. It means compatibility of composite increase. Briefly, it may be seen in Figures 4 and 5.
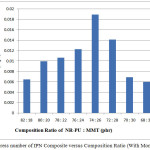 |
Figure 4: Stress number of IPN Composite versus Composition Ratio (With Montmorillonit) |
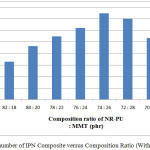 |
Figure 5: Strain number of IPN Composite versus Composition Ratio (With Montmorillonit)
|
Figures 4 and 5 show that composition ratio of NR-PU : MMT 82 : 18 phr has the smallest of stress tensile number(6,48 x 10-3 Mpa), strain tensile number(65,78 %)and Modulus of Elastisity(9,85 x 10-3 Mpa) among the others. It causes amount of montmorillonit is too small. It will affect pressure transition, heterogenic phase will occur and composite will be incompatible.
SEM Analysis
The morphology of IPN composite is characterized by using SEM-EDS (Figures 6 and 7).
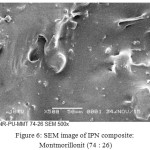 |
Figure 6: SEM image of IPN composite: Montmorillonit (74 : 26) |
Figure 6 show IPN composite’s shape is homogeny among natural rubber, polyurethane andmontmorillonit.Filler may affect stress and strain tensile due to there is surface interface binding. Surface of IPN composite is void and matrix cracking. It is caused effect of strain and stress tensile (Figure 6). There is a rapture and rigid area, indicating by homogeny of matrix phase. In addition, particles of montmorillonit are be well dispersed and inserted into natural rubber and polyurethane composite. Fortunately, montmorillonit particles which are be well inserted into NR-PU has not be destructed along composite process. It probes that IPN formation is exist. In owing to IPN networking just occur at interface binding.
Image of IPN composite with adding MMT (82: 18 phr) is shown in Figure 7. We may see decreasing effect of MMT to IPN composite.
 |
Figure 7: SEM image of IPN Composite: Montmorillonit (82 : 18) |
Figure 7 show that decreasing amount of MMT will cause stress tensile decreases. It may explain that dispersion of MMT is non-homogeny. Therefore, matrix cannot bind MMT fluentlythereby interface between NR-PU and montmorillonit is weak. That is consistent with debonding at rapture area also decreaseand there is matrix cracking. Debonding occurs when adding montmorillonit decrease. The weak interaction causes stress tensile of IPN composite will be decrease as amount of filler decreasing effect (Savetiana, 2013). At low concentration of filler, the IPN composite rigidity is weak. It is caused chain formation of composite disperse non-homogeny (Jacob, J. 2010).
In this research, we found that optimize amount of montmorillonit on NR : PU matrix will produce good IPN composite base on mechanic properties. It may be caused compatible occurs at IPN composite. In contrast, too much amount of montmorillonit will be disrupt compatibility of all matrix components thereby the mechanic properties will decrease.
DSC Analysis
DSC data of IPN PU-NR composite is shown in Figure 8.
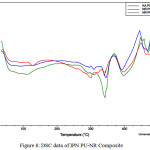 |
Figure 8: DSC data of IPN PU-NR Composite
|
Figure 8 is thermo gram of IPN NR – PUcomposite. DSC thermo grams will give information about transition glass (Tg), melting point (Tm), anddecomposition temperature. Briefly, thermal analysis of IPN NR : PU composite is shown in Table 3.
Table 3: Thermal analysis of IPN NR-PU composite
|
Materials |
Tg(oC) |
Tm(oC) |
Decomposition (oC) |
| NR : PU (80 : 20) |
108,38 |
343,00 |
397,63 |
| NR-PU : MMT (74 : 26) |
122,29 |
363,53 |
391,78 |
| NR-PU :MMT (82 :18) |
120,89 |
340,30 |
344,19 |
Table11 shows Tg of NR-PU (108,38oC), NR-PU-MMT (122,29 oC), and 120 oC probes thatproduction of IPN compositeis similar. NR-PU without montmorillonit has Tg is lower than NR-PU-MMT. It is caused montmorillonit is exist in IPN composite.Indicating IPN NR-PU-MMT compositehas Tg is higher than NR : PU(without MMT).
In addition, Tm of NR-PU : MMT is higher than NR : PU. That is caused IPN exist and it will increase the decomposition temperature of IPN composite.
Shobing (2011) said existence of filler in IPN composite may be increasing thermal stability of NR-PU composite. Transition glass of IPN composite is proportional to homogeny of (heterogenic phase) (Marinovic, S. 2010). Over rate of scanningalso will cause Thermal-lageffect (Yulindo, Y. 2008).
In this paper we propose mechanism reaction of IPN NR-PU-MMTcomposite (Figure 9).
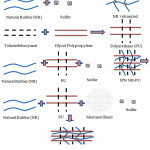 |
Figure 9: Mechanism of IPN compositeFormation |
Conclusions
The best concentration of ratio composite to synthesize of IPN between Polyurethanes-Natural Rubber SIR-10 with the addition of Monmorillonit is at a ratio of 74 phr: 26 phr with a particle size of montmorillonite is average 413.3 ± 39.3 nm
The strainand stress tensile increase with the addition of 20 phr polyurethane at NR: PU (80: 20 phr) Montmorillonit assists to improve mechanic, morphology and thermal properties of IPN composite.
References
- Abu bakar, A.. Effect Of Accelerated Wethering On The Mechanical Properties Of Oil Palm Emty Fruit Bunch Filled UPVC Composites. Iranian Polymer Journal. 2005.,14 (7) : 627-635.
- Adnanda, D. N.. Pembuatan dan Karakterisasi Komposit Karet Alam/Montmorillonit Menggunakan CTAB Sebagai Pemodifikasi Organik. FMIPA USU : USU Press.2015
- Akram, D.. Synthesis, Characterization And Corrosion Protective Properties OfBoron-Modified Polyurethane From Natural Polyol. Progress In Organic Coatings. 2008.,63.,25-32.
CrossRef - Amrollahi, M. 2011. Investigation Of Novel Polyurethane Elastomeric Networks Based On Polybutadiene-ol/Polypropyleneoxide Mixture and Their Structure–Properties Relationship. Materials and Design. 2011.,32 : 3933–3941.
CrossRef - Andriyanti, W.. Kajian Metode Vulkanisasi Lateks Karet Alam Bebas Nitrosamin dan Protein Alergen. Yogyakarta : Pusat Teknologi Akselerator dan Proses Bahan.2010
- Antonius, P.. Penyediaan Film Mikrokomposit PVC Menggunakan Pemastis Stearin Dengan Pengisi Pati dan Penguat Serat Alam. Tesis Magister. Medan : Program Pasca Sarjana Universitas Sumatera Utara. 2009
- Bandrup. Hand Book Of Polymer. New York : John Wiley and Sons.1985.
- Cahyana, A. 2014. Analisa SEM (Scanning Electron Microscope) Pada Kaca TZN Yang Dikristalkan Sebagian. Prosiding Mathematics and Sciences. ISBN : 978-602-0960-00-5.
- Dana, J.D.1960. The System Of Mineralogy. Volume 2. Edisi 7. New York : Jhon Wiley andSons.
- Ferrer, M.C.C.. Characterisation Of Polyurethane Networks Based On Vegetable Derived Polyol. Polymer.2008., 49 (2008) : 3279-3287.
- Franklin, D. 2013. Analisis Sifat Mekanik dan Daya Serap Air Material Komposit Serat Rotan. Yogyakarta : Fakultas Matematika dan Ilmu Pengetahuan Alam Universitas Negeri Yogyakarta.
- Frederic, V.. Influence Of The Poly(ethylene oxide)/Polybutadiene IPN Morphology On The Ionic Conductivity Of Ionic Liquid. European Polymer Journal. 2013.,49 (2013) : 2670 – 2679.
- Hartomo, A. J. 1996. Memahami Polimer dan Perekat. Yogyakarta : Penerbit Andi Offset.
- Hentschel, T..Kinetics Of The Molar Mass Decrease In A Polyurethane Melt: A Rheological Study. Polymer. 2001.,42 (2001) : 3195-3203.
- Hepburn, C..Polyurethane Elastomer. Second Edition. New York : Elsevier Applied Science.1991
- Hummel, D. O..Infrared Spectra Polymer In Medium and Long Wave Length Region. London : John Wiley and Sons.1985
- Irawati, F. Pengaruh Ukuran Serbuk Tempurung Kelapa Sebagai Pengisi Komposit Poliester Tak Jenuh Terhadap Sifat Mekanik dan Penyerapan Air. Jurusan Teknik Kimia USU.2013.2, 4 (2013).
- Jacob, J. 2010. Main Chain And Segmental Dynamics Of Semi Interpenetrating Polymer Networks Based On Polyisoprene And Poly(methyl methacrylate). Polymer. 2010.,512390-2402.
- Laurent, J. G. Soft and Fexible Interpenetrating Polymer Networks Hosting Electroreflective Poly(3,4-ethylenedioxythiophene). Solar Energy Materials & Solar Cells. 2014.,12., 33–42.
- Marinovic, S.. The Influence Of Different Components On Interpenetrating Polymer Network’s (IPN’s) Characteristics as Automotive Top Coats. Progress in Organic Coatings. 2010.,68.,293–298.
CrossRef - Misra, A.. Swelling Equilibrium Of Dentin Adhesive Polymers Formed On The Water-Adhesive Phase Boundary: Experiments And Micromechanical Model. Acta Biomaterialia. 2014.,10.,330-342.
CrossRef - Morton, M.. Rubber Technology. Third Edition. New York : Van Nostrand Reinhold.1987
CrossRef - Nicolas, F. Electro-Active Interpenetrating Polymer Networks Actuators and Strain Sensors : Fabrication, Position Control and Sensing Properties. Sensorsand Actuators B. 2014.193 (2014) : 82–88.
- Odian, G.. Principles Of Polymerization. Fourth Edition. New Jersey : John Wiley & Sons, Inc.2004
CrossRef - Ompusunggu, M. Pengawetan Bahan Olah Lateks Kebun. Warta Perkaretan. Medan: Pusat Penelitian Perkebunan.1987.
- Pal, K.. Effect Of fillers On Morphological And Wear Characteristics Of NR/HSR Blends With E-Glass fiber. Material and Design. 2012.,35 (2012) : 863-872.
- Ramos, B.G.Z. 2006. Polyurethane Nanoparticles From A Natural Polyol Via Miniemulsion Technique. Polymer. 47 (2006) : 8080-8087.
CrossRef - Rohaeti, E. Kajian Tentang SintesisPoliuretan dan Karakterisasinya. Yogyakarta: Fakultas Matematika dan Ilmu Pengetahuan Alam Universitas Negeri Yogyakarta.2005.
- Rohaeti, E.. Analisis Sifat Termal Poliuretan Berbasis Minyak Jarak dan Toluena Diisosianat Dengan DTA dan TGA. Yogyakarta : Fakultas Matematika dan Ilmu Pengetahuan Alam Universitas Negeri Yogyakarta. 2011
- Rosado, E.D.. Thermal Degradation Of Urethane Modified Polyisocyanurate Foamsbased On Aliphatic And Aromatic Polyester Polyol. Polymer Degradation and Stability. 2002.,78 (2002) : 1-5.
- Rusdi, R.. Karakteristik Matriks Termoplastik Polietilena Terplastis Poligliserol Asetat. Tesis Magister. Medan : Program Pasca Sarjana Universitas Sumatera Utara.2008
- Saelao, J. Influence Of Styrene on Grafting Efficiency Of Maleic Anhydride Onto Natural Rubber. Bangkok : Department Of Chemistry, Faculty Of Science, Mohidol University.2004.
- Savetina, S.. Kekuatan Tarik Komposit Poliester Berpenguat Partikel Kayu Jati, Merawan dan Meranti Merah. Jurnal Mechanical.,2013.,4. 1.
- Septiari, I.. Pembuatan Papan Partikel Dari Limbah Plastik Polyprophylene (PP) dan Tangkai Bambu. Singaraja. E-Journal Kimia Visvitalis : 2014.,2.,1 Tahun 2014.
- Shoubing, C.. Hydroxy-Terminated Liquid Nitrile Rubber Modified Castor Oil Based Polyurethane/Epoxy IPN Composites : Damping, Thermal and Mechanical Properties. Polymer Testing. 2011.,30 (2011) : 726–731.
- Srilekha, S.D.. Exposure Of Natural Rubber to Personal Lubricants-Swelling and Stress Relaxation As Potential Indicators Of Reduced Seal Integrity Of Non-Lubricated Male Condoms. Milwaukee : Biomedical Engineering, Marquette University.2014
- Stevens, M. P.. Kimia Polimer. Cetakan Kedua. Jakarta : Pradnya Paramita.2007
- Tamrin. Penyediaan dan Pencirian Polimer Jaringan Saling Menembus Antara Getah Asli dan Poliuretana. Malaysia : Universiti Teknologi Malaysia.1997.
- Tatachiwin, L., Sakdapipanich, J., Ute, K., Kitayama, T., Tanaka, Y. Structural Characterization Of Terminal Group Of Natural Rubber 2 : Decomposition Of Branch-points By Phospolipase And Chemical Treatments. Biomacro-molecules.2005.6. 1858-1863.
- Tim Penulis Penebar Swadaya. 1992. Karet : Strategi Pemasaran Tahun 2000 Budidaya dan Pengolahannya. Jakarta : Penebar Swadaya.
- Treloar, L.R.G. The Physics Of Rubber Elasticity. London : Oxford University Press.1958.
- Triwulandari, E.. Studi polimerisasi Antarmuka Terhadap Distribusi Ukuran Partikel Mikrokapsul Poliuretan Berbasis Gliserol. Tesis Magister. Depok : Universitas Indonesia.2012
- Vivek, J. D. Synthesis and Characterization Of Interpenetrating Polymer Networks From Transesterified Castor Oil Based Polyurethane and Polystyrene. Journal of Saudi Chemical Society. Gujarat : Sardar Patel University.2013.
- Wake, W. C.. Synthetic Adhesive and Sealants. New York : John Wiley and Sons.1987
- Wirjosentono, B.. Analisis dan Karakterisasi Polimer. Medan : USU-Press.1995
- Xianwu, Z.. Synthesis And Properties Of Polyurethane Foams Prepared From Heavy Oil Modified By Polyols With 4,4’-Methylene-Diphenylene Isocyanate (MDI). Bioresource Technology.2012.,114 (2012) : 654-657.
- Yulindo, Y.. Migrasi Dioktil Ftalat dan Etilen Glikol ke Dalam Struktur Poliuretan Dengan Pemanjangan Rantai Diamina Aromatik dan Pengaruhnya Terhadap Kinerja Material. [Tesis]. Jakarta : Universitas Indonesia.2008
- Zhang, L.. Substituting Soybean Oil-Based Polyol Into Polyurethane flexible Foams. Polymer. 2007.,48 (2007) : 6656-6676.

This work is licensed under a Creative Commons Attribution 4.0 International License.









