Composite Fe3O4@Au Core-Shell Nanoparticle: Tunable and Enhancement of Optical Absorption Property
Artit Chingsungnoen1, Panuwat Chaiyachate2 and Thananchai Dasri3
1Technological Plasma Research Unit, Department of Physics, Faculty of Science, Mahasarakham University, 44150, Thailand.
2School of Science and Mathematics, Faculty of Industrial and Technology,Rajamangala University of Technology Isan SakonNakhon Campus, SakonNakhon, 47160, Thailand.
3Faculty of Applied Science and Engineering, Khon Kaen University, Khon Kaen, 40002,Thailand.
Corresponding Author E-mail: thananchai_dasri@hotmail.com
DOI : http://dx.doi.org/10.13005/ojc/330407
Bifunctional nanomaterial Fe3O4@Au core-shell is a kind of nanoparticle that includes magnetic iron oxide core with gold coated. It can be used in can achieve the controllable and tunable electromagnetic fieldenhancement and may open up exciting opportunities toengineer new materialsused in biomedical applications.This research workaims at theoretical investigating the basic optical absorption property of Fe3O4@Au core-shell with varying number particles, inter-particle distance, and direction of incident lightusing the discrete dipole approximation method (DDA) in thewavelength of visible to near-infrared ranges of theelectromagnetic wave spectrum.The results were observed that these parameters can beenhanced optical light absorption and tuned the LSPR peak position. The obtained results might be used for basic fabricating the more performance specific applications like adrug in life science.
KEYWORDS:Plasmonic nanoparticles; Fe3O4@Au core-shell; discrete dipole approximation
Download this article as:| Copy the following to cite this article: Chingsungnoen A, Chaiyachate P, Dasri A. Composite Fe3O4@Au Core-Shell Nanoparticle: Tunable and Enhancement of Optical Absorption Property. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Chingsungnoen A, Chaiyachate P, Dasri A. Composite Fe3O4@Au Core-Shell Nanoparticle: Tunable and Enhancement of Optical Absorption Property. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35154 |
Introduction
Nanostructured materials which at least one dimension the particle size is between 1 nm to 100 nmhave been attracted great attention ofscientists due to their propertiesincluding optical, magnetic, specific heat, melting point, surface activities, chemical and biological properties are highly size dependent compared to their bulk counterparts. Also, iron oxide nanomaterial of magnetite (Fe3O4) which its particle size smaller than 20 nmcan be considered in the rangeof a single domain and exhibits a superparamagnetic property1-2. A magnetic resonance imaging (MRI), medical imaging technique using a strong magnetic fieldandradio waves for body diagnosis, is a major application2-3. Moreover, typical metal nanoparticles (NPs) of gold (Au) and silver (Ag)have great attracted in many applications4-8due to exhibiting of surfaceplasmon (SP) phenomenaandopticalactivity. SP is electromagnetic (EM) wavescoupledto the collective oscillations of the conduction electron in aninterface between two media with permittivities with oppositesign such as between metal and dielectric. It can be either propagating called surface plasmonpolaritons (SPPs) or propagating surface plasmons, in theplanar bulk metal surface or localizedsurface plasmon(LSP), in the case of NPs.At certainly excited photon energy, the collective oscillations ofthis electron interact the most efficiently with the incident photon, result in a maximum light absorption. This is defined as localized surface plasmon resonance (LSPR). However,nanomaterials consisting of single component usually contain only one unique property of one active in gradient. Therefore, the intense research in the development of nanoparticles (NPs)that combine multiple functions orproperties not obtainable in individualmaterialsis motivatedby the scientists to obtain new materials inorder to further many applications. Also, nanoparticles composed of aFe3O4core, well known as magnetic properties,and continuous plasmonic (Au or Ag) shell layers, non-magnetic material but shown SP properties, for example, are of increasing interest for biomedicine application 9-12.This article the only optical absorption property in multicomponent Fe3O4@Au core-shell nanoparticle embedded in water will be theoretically investigated. The surface plasmon resonance was indicated that it can be enhanced and tuned by adjusting the inter-particle distanceand number of particlesin the particle-chain. This study will contribute to better understanding about the optical properties which should be obtained from the construction or synthesis the Fe3O4@Au core-shell bifunctionalnanostructures for the associated applications.
Experimental
Computational Details
The optical absorption efficiencies of the composite Fe3O4@Au core-shell nanoparticle werecalculated based on the DDA theory13. This theory, the object in arbitrary shape is replaced with an assembly ofN point dipoles in which the polarizability and positions are specified as aiandri, respectively. The polarization inducedPi in each particle in the presence of an applied field is then described by Eq.(1):
![]()
where Eloc is the sum of the incident field and the contribution from all other N-1 dipoles. When this polarization is obtained, the absorption, scattering and extinction cross sections of light can be calculated by Eqs. (2)- (4):
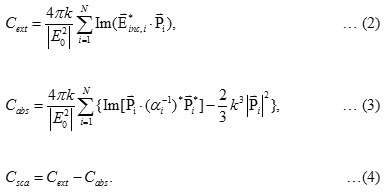
Equations (2) and (3), ‘*’ symbol stands for the conjugate of a complex variable, kis the wave number and E0 is the amplitude of incident electric field.And scattering, absorption and extinction efficiencies can be obtained by divided the optical cross section by their cross section area of the particle. For the composite nanoparticle assumed to be spherical, with a Fe3O4 core (εc) of radius b, surrounded by a shell of gold (εb) to outer radius a, much less than the wavelength of light being considered. The composite particle’s dielectric function εs is found to be14

where fc= (b/c)3 is the internal volume fraction. Eventually, the absorption cross sections can be calculated using equation (3). In this calculation, the dielectric constants for Fe3O4 were taken from Ref.15.The Fe3O4 @Au core-shell nanoparticle size was taken from Lim and co-workers9. The dielectric constants for metal NPswereextracted from the experimental results in Ref.16. The dielectric constant of water which was used as the surrounding medium due is 1.77.
Results and Discussion
The calculated absorbance spectra of bimetallic nanoparticles (NPs)of Fe3O4@Au core-shell NP with the 18 nm Fe3O4 core of size coated by 5 nm Au layer thick embedded in water are presented. First, in Fig. 1, we show theabsorbance spectra of Fe3O4@Au core-shell NP in thewavelength range between 350 nm to 800 nm compared with the experimental results, Fe3O4@Au core-shell NP with the same condition and pure Fe3O4 NP (adapted from ref. 9). Our calculations found that the LSPR peak position was agreement with the experimental data. Thepeak position exhibited at ~602 nm which is in the visible wavelength region of electromagnetic (EM) wave. On the other hand, absorption peak for the single nanomaterial of pure Fe3O49does not obviously show any absorptionin the visible region (350-800 nm).Later, in Figs. 2 and 3, the absorption efficiency of Fe3O4@Au core-shell NP size and inter-particles distance were fixed, but the number of particles was varied between two particles to four particles. Fig. 2 show the absorption efficiency of the Fe3O4@Au core-shell NPs aligned perpendicular to the propagation direction and parallel to the polarization direction of light.On the other hand in Fig. 3, the Fe3O4@Au core-shell NPs axiswas aligned perpendicular to both the propagation direction and the polarization direction of light.First observation, by compared with the pure Fe3O4NP that was not obviously found the LSPR peaks, the absorption efficiency can be enhanced after coating plasmonic particle of Au nanolayeron theFe3O4core. In addition for in Fig. 2, it was observed thatthe increasing of the number particles from 1 particle to 3 particles the LSPR peak position shifts to thered light invisible region of EM wave compared with Au spectrum (LSPR peak position at ~ 520 nm).The LSPR peak positions were strongly observed at ~ 602 nm, ~ 686 nm and ~ 786 nmfor nanostructured of 1 particle, 2 particles, and 3 particles, respectively.Whereas aligning particles axis perpendicular to both the propagation direction and thepolarization direction of light, blue-shifted was observed as seen in Fig. 3.Last calculation, shown in Fig. 4, the absorption spectra obtained from the closely two Fe3O4@Au core-shell NPs were calculated. In the calculation, these particles (fixed particles size) were aligned on the x-axisperpendicular to the propagation direction and parallel to thepolarization direction of light.The inter-particles distance was varied between 12 nm to 100 nm. The obtained results were observed that the LSPR peak position was found at~1080 nm for 12 nm of particles distance and shifts to shorter wavelengthof ~820 nm, ~734 nm, ~692 and ~602 nm as the inter-particles distance changed to13 nm, 14 nm, 15 nm and 100 nm, respectively. And it is clearly in Fig. 5 that shows the LSPR peak as afunction of the inter-particles distance. It was found that the absorption peak position shifts to shorter wavelength nonlinearly with exponential-like decrease function.The shifted resonance peak position can be qualitative explained17 that first due to the object in arbitrary shape in DDA model is replaced with an assembly of N point dipoles. Therefore, the aligning particles axis parallel to the polarized light direction the plasma electron distributions of all particles is therefore perturbed with theweakening of the repulsive forces of the field. This effect leads to a correspondingly lower resonance frequency or longer wavelength as seen in the simulated results Figs. 2. In contrast, when the excited electric field incidents normal to the longer particles axis the charge distributions of each particle acts cooperatively to enhance the repulsive action in all particles, thus decreasing the resonance wavelength as seen in Figs. 3.The secondexplanation is the plasmon modes coupling theory18 which two distinct plasmon modes of an outer-shell surface sphere mode and an inner-shellsurface cavity mode of Au shell are produced. Therefore, the splitting into two new modes of higher frequency plasmon mode and lower frequency plasmon mode is induced. This phenomenon influences the changing of LSPR peak position. It iswellknown that the spectral location of the LSPR is sensitive to the change of the environmental refractive index. Consequently, the high refractive index, ~3-5 in the visible region, the effect of Fe3O4on the SPR frequency is large, leading to the enhancement of absorption spectra.The obtained results were found that it should be possible to shift the LSPRs frequency into the near-infrared region (NIR) of the spectrum by increase the number particle and inter-particles distance as seen in Fig. 2 and Fig. 3, respectively.
![Figure 1: Comparison between theoretical calculation and experimentally obtained UV-Visible spectrum (adapted from Ref. [*]) for a particle with an 18 nm Fe3O4 core and 5 nm thick gold shell in water.](http://www.orientjchem.org/wp-content/uploads/2017/07/Vol33No4_Comp_Arti_fig1-150x150.jpg) |
Figure 1: Comparison between theoretical calculation and experimentally obtained UV-Visible spectrum (adapted from Ref. [*]) for a particle with an 18 nm Fe3O4 core and 5 nm thick gold shell in water. |
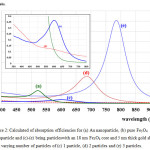 |
Figure 2: Calculated of absorption efficiencies for (a) Au nanoparticle, (b) pure Fe3O4 nanoparticle and (c)-(e) being particleswith an 18 nm Fe3O4 core and 5 nm thick gold shell with varying number of particles of (c) 1 particle, (d) 2 particles and (e) 3 particles. Click here to View figure |
The particles axisis perpendicular propagation but parallelto the polarization direction of light.
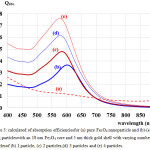 |
Figure 3: Calculated of absorption efficienciesfor (a) pure Fe3O4 nanoparticle and (b)-(e) being particleswith an 18 nm Fe3O4 core and 5 nm thick gold shell with varying number of particlesof (b) 1 particle, (c) 2 particles,(d) 3 particles and (e) 4 particles. |
The particles axisis perpendicular to both the polarization and propagation direction of light.
 |
Figure 4: Calculated absorption efficiencies in dependence of excited wavelength for two particlesof an 18 nm Fe3O4 core and 5 nm thick gold shell with varying the inter-particles distance, (a) 100 nm, (b) 15 nm, (c) 14 nm, (d) 13 nm and (e) 12 nm, aligned on x-axis. |
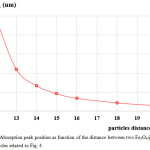 |
Figure 5: Absorption peak position as function of the distance between two Fe3O4@Au core-shell particles related to Fig. 4. Click here to View figure |
Conclusion
In summary, the optical absorption property of the Fe3O4@Au core-shell NPs with varying their number particle, inter-particle distance and direction of polarization and propagation of incident light were studied using the DDA method. The results found that the coating of Au nanolayer on aFe3O4spherical nanoparticle in the water which is the main component solution in the human body shows strongly LSPR induced. Moreover, the results revealed that the resonance peak of LSPR can be tuned over wavelength from the visible tothe near-infrared region of the spectrum.Thus, the basic results of this study might be used for basic fabricating the more performance specific applications like adrug in life science.
Acknowledgments
The authors are grateful to the Faculty of Applied Science and Engineering, KhonKaen University for all facilities supported.This work was partially supported by the Nanotechnology Center (NANOTEC), NSTDA, Ministry of Science and Technology, Thailand through its Center of Excellence Network program.
References
- Goya, G. F.;Berquo, T. S.; Fonseca, F. C.J Appl. Phys.2003, 94, 3520–3528.
CrossRef - Sheng-Nan, S.; Chao, W.; Zan-Zan, Z.; Yang-Long, H.; Venkatraman, S. S.; Zhi-Chuan, X. Chin. Phys. B 2014, 23,037503
CrossRef - Arbab, A. S.; Bashaw, L. A.; Miller, B. R.; Jordan, E. K.; Lewis, B. K.;Kalish H.; Frank, J. A.Radiology2003, 229, 838–846
CrossRef - Khlebtsov, N. G.; Dykman, L. D. J. Quant. Spectrosc. Radiat.Transf.2010, 111, 1-35
CrossRef - Liu, F.;Xie, W.; Xu, Q.; Liu, Y.; Cui, K.; Feng, X.; Zhang, W; Huang, Y.IEEE Photonics J. 2013, 5, 8400509
CrossRef - Cheng, C.-E.; Pei, Z.; Hsu, C.-C.; Chang, C.-S.; Chien, F. S.-S.Sol. Energy Mater. Sol. Cells2014, 121, 80-84
CrossRef - Notarianni, M.; Vernon, K.; Chou, A.;Aljada, M.; Liu, J.; Motta, N.Sol. Energy2014, 106, 23-37
CrossRef - Tang, M.; Zhu, W.; Sun, L.; Yu, J.; Qian, B.; Xiao, T. Synth. Met.2015, 199, 69-73
CrossRef - Lim, J.;Eggeman, A.;Lanni, F.; Tilton, R. D.; Majetich, S. A. Adv. Mater. 2008, 20, 1721–1726
CrossRef - Lim, J.;Majetich, S. A. Nano Today2008, 20, 98–113
- Banerjee, M.; Sharma, S; Chattopadhyay, A; Ghosh, S. S. Nanoscale2011,3, 5120-5125
CrossRef - Laura Rodriguez-Arco,L.; Rodriguez, I. A.; Carriel, V.; Bonhome-Espinosa, A. B.; Campos, F.; Kuzhir, P.; Duranab, J. D. G.; Lopez-Lopez, M. T. Nanoscale2016,8, 8138-8150
CrossRef - Purcell, E.M.; Pennypacker, C. R. Astrophys J. 1973, 186, 705-714
CrossRef - Dani, R. K.; Wang, H.; Bossmann, S. H.;Wysin, G.; Chikan, V. J. Chem. Phys.2011, 135, 224502.
CrossRef - Fontijn, W.F.J.; van der Zaag, P.J.;Devillers, M.A.C.;Brabers, V.A.M.; Metselaar, R.Phys. Rev. B1997, 56, 5432-5442
CrossRef - Johnson, P. B.; Christy R. W. Phys. Rev. B. 1972, 6, 4370–4379.
CrossRef - Rechberger, W.; Hohenau, A.; Leitner, A.; Krenn, J. R.; Lamprecht, B.; Aussenegg, F. R. Opt.Commun.2003, 220, 137–141
CrossRef - Prodan, E.; Nordlander, P. J. Chem. Phys.2004, 120, 5444-5454.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









