Calcium Ferrite, an Efficient Catalyst for Knoevenagel Condensation (A Green Approach)
Parveen Pippal and Prabal Pratap Singh
Department of Chemistry, GLA University, 17 km stone, NH-2 Delhi-Mathura highway, Chaumuhan Mathura 281406 UP India.
Corresponding Author E-mail: prabal.singh@gla.ac.in
DOI : http://dx.doi.org/10.13005/ojc/330418
Calcium ferrite NPs catalyst have been used as a cheaper and highly efficient catalyst for Knoevenagel condensation of active methylene substrate with various carbonyl compounds affording condensed products in excellent yields in shorter reaction time. The developed greener protocol is very simple, involving cleaner work up and the synthesized products do not require further purification. The catalyst can easily be removed and reused at least for four times without any appreciable change in reactivity.
KEYWORDS:Knoevenagel condensation; Calcium ferrite; Ethylcyanoacetate; Malononitrile; Reusable catalyst; Magnetic nano particle; Magnetic Separation
Download this article as:| Copy the following to cite this article: Pippal P, Singh P. P. Calcium Ferrite, an Efficient Catalyst for Knoevenagel Condensation (A Green Approach). Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Pippal P, Singh P. P. Calcium Ferrite, an Efficient Catalyst for Knoevenagel Condensation (A Green Approach). Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35552 |
Introduction
Knoevenagel condensation is a very useful reaction in organic chemistry known for its synthetic utility in carbon carbon bond formation [1] and their enormous applications particularly in fine chemicals [2] and therapeutic and pharmacological products [3-5]. The condensation reaction is generally performed by using stoichiometric or catalytic amounts of ammonia, primary and secondary amines, pyridine, piperadine [6] dimethyl amino pyridine [7-10] Al2O3 [11], SmI3 [12], CdCl2 [13], LaCl3 [14], ZnCl2 [15], Zeolite [16], ZnCl4-SiO2 [17], fluorapatite [18], hydroxyapatite [19], metal oxides [20], ionic liquids like [Bmim]Cl.xAlCl3 [21], [MMIm][MSO4] [22], ethylenediammonium diacetate in ionic liquids [23], hydrotalcite in ionic liquids [24], mesoporous organosilica supported potassium carbonate (BPMO-IL-KCO3) [25], UiO-66-NH2 [26]. In addition, there are certain reports on the use of electrochemical induced, microwave and ultra sound irradiation methods for Knoevenagel condensation [27-28]. Recently nano particles like BaAl2O3 [29], Fe3O4@SiO2 [30], P4VP/Al2O3-SiO2 [31], Nitridated KCC-1 [32], amine functionalized PAN [33], Pd(0) [34] has been reported to catalyze the Knoevenagel condensation reaction. Various MOF [35-37] has also been tried to catalyze the above reaction. However these methods suffer from disadvantages such as unsatisfactory yields, long reaction times, stoichiometric amount of catalyst, harsh reaction conditions, use of hazardous solvents, non recoverable catalyst that contains toxic metals, moisture sensitive nature of catalyst and tedious work up procedure. Therefore there is a great need to design and develop a protocol that does not have the above problems. Here in this paper we report a greener approach towards synthesis of Knoevenagel condensation products using a highly efficient, cheaper, reusable, biocompatible and environment friendly heterogeneous nano catalyst yielding the products in good to excellent yields in shorter reaction times. The developed protocol involves cleaner and easier work up procedure reducing the use of hazardous solvents.
Materials and Methods
Experimental
General Information
All the solvents and reagents were used as such as supplied from commercial sources. The recorded melting points are uncorrected. IR spectra were recorded in KBr on a Schimadzu FTIR 8401 spectrometer and Perkin Elmer version10.03.06 for the liquid samples. 1H and 13C spectra were recorded on a Bruker DRX 300 spectrometer operating at 300 MHz for 1H NMR and 75 MHz for 13C NMR as solutions in CDCl3 and DMSOd6. The ESI mass spectra were measured on waters UPLC-TDQ spectrometer. TLC was performed on silica coated glass plate, spots were developed in I2 Chamber or visualized in UV chamber. SEM was recorded at Technai G2 30S TWIN at 300KV accelerating voltage. Powder X-Ray Diffraction (PXRD) pattern was recorded on Hecus X-Ray Systems S3 Model using Cu Kα radiation (k = 1.5404 A) from 0-10°.
Preparation of Calcium Ferrite NPs Catalyst
In a typical procedure for the synthesis of catalyst the mixture of calcium carbonate (1 equiv), sugar (17 equiv), Fe2(NO3)3.9H2O (2 equiv), monoethanolamine (25 equiv), HNO3 (44 equiv) and double distilled water (300 mL) in a beaker was heated on hot plate up to the dryness. The black residue obtained was kept in preheated muffle furnace at 800 ± 10°C for 6-7 hrs. The resulting brown colored powder was further characterized by XRD (Fig.1), SEM (Fig. 2) and FTIR (Fig. 3) in order to determine their formation, size and morphological behavior.
General Procedure for Knoevenagel Condensation Reaction (3a-M)
To a well stirred reaction mixture of aldehyde (1mmol) and active methylene compound (1mmol), in 10 mL methanol, the calcium ferrite (5 mmol%) catalyst was added and refluxed for the requisite period of time. The progress of reaction was monitored by TLC. On completion of the reaction the catalyst was removed with the help of external magnet. The supernatant liquid was decanted and reaction mixture was concentrated on vacuum rotary evaporator to obtain crude material which was then recrystalized using EtOAc: hexane mixtures. In general, no further purification was required for solid product. However for liquid mixture, the crude product was purified by column chromatography over silica gel (100-200 mesh size) to get the pure compound. All products were characterized 1H & 13C NMR spectroscopy, Mass and FTIR spectroscopy (See electronic supporting information). The recovered catalyst was further used to check its number of recyclability and efficacy.
Characterization of Catalyst
The XRD spectrum of CaFe2O4 nano particle is presented in Fig 1 showing the lattice and structural parameters of catalyst.
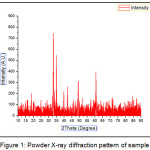 |
Figure 1: Powder X-ray diffraction pattern of sample |
The morphology of the nano particle was investigated using SEM analysis (Fig 2). The micrograph reveals that the particles are elongated agglomerated having uneven size and distribution. Agglomeration may be due to the magnetic dipole interaction between ferrite particles. The particles have large number of voids due to release of gaseous products. The size of the particles ranges from 100-150nm.
 |
Figure 2: SEM micrographs at different magnifications (A-C) |
The FT-IR absorption spectrum of sample calcinated at 800˚C for 4 hrs in presented in fig 3. Strong absorption bands at 670 and 478 cm-1 can be assigned to the stretching vibration frequency of Fe-O bands.
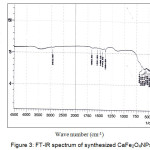 |
Figure 3: FT-IR spectrum of synthesized CaFe2O4NPs Click here to View figure |
Catalytic Activity
In connection with our enduring interest concerning the investigation of new heterogeneous CaFe2O4 nano catalyst, we here in report a highly effective and economic protocol for Knoevenagel condensation using calcium ferrite catalyst in methanol as a solvent (Scheme 1). The strategy was then applied for the condensation of various aldehydes and active methylene compounds to yield knoevenagel products.
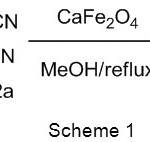 |
Scheme 1 |
Result and Discussion
In our preliminary study, benzaldehyde 1a was treated with malononitrile 2a in presence of various catalysts taking methanol as solvent (Table 1). The expected knoevenagel condensed product 2-benzylidenemalononitrile 3a could not be isolated even in trace amount in absence of catalyst (Table 1, entry 1). It was observed that CaFe2O4 NPs catalyzed reaction completed within 20 min to give the product 3a in 95% yield (Table 1, entry 8). However other catalysts viz Fe2O4 and SrFe2O4 could not initiate the reaction even after prolonged refluxing (Table 1, entries 2-3), where a few catalysts were able to generate 3a in very less yields (Table 1, entries 4-7).
Table 1: Screening of catalysts for Knoevenagel condensation product
|
Entry |
Catalysta |
Time (min) |
Yieldsb |
|
1 |
No catalyst |
120 |
NP |
|
2 |
Fe2O4 |
120 |
NP |
|
3 |
SrFe2O4 |
120 |
NP |
|
4 |
ZnO@Fe2O4 |
120 |
15 |
|
5 |
CuFe2O4 |
120 |
10 |
|
6 |
ZnFe2O4 |
120 |
20 |
|
7 |
MgSO4 |
120 |
25 |
|
8 |
CaFe2O4 |
20 |
95 |
aReaction conditions: benzaldehyde (1mmol); malononitrile (1mmol); methanol 10mL; reflux bIsolated yields
Inspiring by the above results we then tried to optimize the reaction condition by treating 1a and 2a with CaFe2O4 NPs using different solvents (such as toluene, xylene, water, ethanol, acetonitrile and DMF). The results are shown in table 2. The yields of the products for reaction in presence of toluene, xylene, water, acetonitrile and DMF as solvent were lower than that of observed in methanol at refluxing condition (Table 2, entry 2). Although ethanol gave comparable yield (Table 2, entry 4).
Table 2: Solvent effects on the reaction of 1a with 2a under calcium ferrite as catalyst
|
Entry |
Solventa |
Time (min) |
Yield (%)b |
|
1 |
Toluene |
120 |
50 |
|
2 |
MeOH |
20 |
95 |
|
3 |
MeCN |
60 |
60 |
|
4 |
EtOH |
20 |
92 |
|
5 |
DMF |
60 |
40 |
|
6 |
Water |
120 |
65 |
aReaction conditions: benzaldehyde (1mmol); malononitrile (1mmol); CaFe2O4 (10 mmol%); refluxing temperature; bIsolated yields
To study the effect of catalyst loading, the knoevenagel condensation was carried out with varying amount of catalyst taking the model reaction (Scheme 1). The desired product was found to show the best yields at 10 mmol% of the catalyst (Table 3, entry 5). While excess of the catalyst loading could not improve the yield further (Table 3, entries 6-7).
Table 3: Study of catalyst loading on the Knoevenagel condensation
|
Entry |
Catalyst (mol %)a |
Yield (%)b |
|
1 |
1 |
40 |
|
2 |
2 |
65 |
|
3 |
3 |
70 |
|
4 |
5 |
80 |
|
5 |
10 |
95 |
|
6 |
15 |
94 |
|
7 |
20 |
94 |
aReaction conditions: benzaldehyde (1mmol); malononitrile (1mmol); time 20 min;methanol reflux: bIsolated yields
Interestingly calcium ferrite nano particle catalyzed reactions were found to be very clean and the products were obtained in extremely pure crystalline form with an average yield of 80-96%. All the reactions were completed in very less time of 10-45 min. Under the optimized reaction condition the generality of the protocol was investigated with various aromatic and indolyl aldehydes with different active methylene substrates. The results are summarized in table-4.
 |
Table 4: Knoevenagel condensation of various aldehydes and active methylene compounds in presence of calcium ferrite NPs. |
Calcium ferrite MNPs catalysed reactions were found to be very clean and the products obtained in pure crystalline form while aliphatic condensed products were viscous oily and were purified by passing through column chromatography.
In general, knoevenagel condensation reactions of various carbonyl compounds with malononitrile were faster (Table 4, entries 1,3-5,7-8 and 10-13) than ethylacyanoacetate (Table 4, entries 2,6 and 9). This may be attributed to the strong electron withdrawing nature of nitrile groups compared to ester group. However reactions involving aldehydes bearing electron withdrawing groups were faster and high yielding (Table 4, entries 7-10) as compared to electron donating groups (Table 4, entries 1-6). The procedure was successfully applied to various substituted aromatic aldehydes with malononitrile and ethylcyanoacetate (Table 4). The ether (entries 3-6) and esters (entries 2,6 and 9) linkages in aromatic aldehydes were remain unaffected in warmer alcoholic conditions. Heteroaromatic aldehyde also gave 3k in very good with malononitrile (Table 4, entry 11). The reaction was further investigated for the synthesis of p-bis-2-(phenylidene)malononitrile by the condensation of terephthaldehyde with two moles of malononitrile to afford the desired product 3l in 95% yield.(Table 4, entry 12).
Reusability of Catalyst
Interestingly we have noteworthy examined the recovery and reuse of the catalyst (Fig 4). After the product formation, the catalyst was recovered with the help of strong external magnet and the supernatant liquid was decanted. The catalyst was washed with excess of methanol, dried in oven for 4 hours and finally used for further runs. The heterogeneous CaFe2O4 MNPs substantially demonstrated the ability to retain its catalytic activity even for four cycles Fig 4.
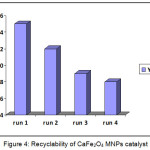 |
Figure 4: Recyclability of CaFe2O4 MNPs catalyst |
The plausible mechanism for the formation of the products (3a-m) has been shown in scheme 2. It is assumed that Ca NPs facilitate the knoevenagel type coupling by co-ordinating to oxygen of carbonyl groups. Also Ca NPs can activate malononitrile or ethylcyanoacete so that deprotonation occurs. As a consequence knoevenagel condensation proceeds by activation of reactants by NPs.
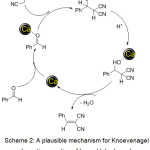 |
Scheme 2: A plausible mechanism for Knoevenagel condensation reaction of benzaldehyde and malononitrile over CaFe2O4 NPs catalyst. |
Conclusion
The synthesized calcium ferrite MNPs act as heterogeneous catalyst that may be practical alternate for application of knovenagel reaction in the view of the following advantages a) high catalytic activity under milder condition, b) easier and cleaner external magnetic separation, c) waste minimization without any side reaction, d) use of non-toxic and inexpensive catalyst, e) reusability of catalyst, d) biocompatibility and environmental friendly catalyst due to presence of Ca2+ instead heavy metal.
Supporting Information
For analytical/spectral data see the attached supporting file.
Acknowledgements
PP and PPS sincerely thanks Chancellor of GLA University for the financial support and infra structure.
References
- Trost B. M. Comprehensive Organic Synthesis, Pergamon Press, Oxford, 1991, vol. 2, p 133-340. Knoevenagel, E. Chem. Ber. 1898, 31, 2585-22595.
- Freeman, F. Chem. Rev., 1980, 80, 329-350.
CrossRef - Jones, G. Org. React. 1967, 15, 204-599.
- Santamarta, F.; Verdia, P.; E. Tojo Catal. Commun. 2008, 9, 1779-1781.
- Ranu, B. C.; Jana, R.; Sowmiah, S. J. Org. Chem. 2007, 72, 3152-3154.
CrossRef - Reddy, T. L.; Varma, R. S.; Tetrehedron Lett. 1997, 38, 1721.
CrossRef - Kidwai, M.; Sapra, P.; Bhusan, K. R. J. Indian Chem. Soc. 2002, 79, 596.
- Dave, C. G.; Auguestine, C. Ind. J. Chem. 2000, 39, 403.
- Tu, S. J.; Rong. L. C.; Gao, Y.; Guo, S. M.; Lu, S. H.; Yang, X. J.; Chin. J. Org. Chem. 2002, 22, 364.
- Narsaiah, A. V.; Basah, A. K.; Visali, B.; Nagaiah, K. Synth Commun. 2004, 34, 2893.
CrossRef - Texiety-Boullet F.; Foucaud, A Tetrahedron Lett. 1996, 26, 3025.
- Bao, W.; Zhang, Y.; Wang, J. Synth. Commun. 1996, 26, 3025-28
CrossRef - Atranshi, O. Fillippone, P.; Mel, A. Synth. Commun. 1998, 13, 1203-1207.
- Narsaiah, A. V.; Nagaiah, K.; Synth. Commun. 2003, 33, 3825-3832.
CrossRef - Rao, P. S.; Venkatraman, R. V.; Tetrahedron Lett. 1991, 32, 5821-5824.
CrossRef - Tu, S. J.; Rong, L. C.; Gao, Y.; Guo, S. M. Lu, S. H.; Yang, X. J. Chin. J. Org. Chem. 2002, 22, 364.
- Vijender, M.; Kishor, P. Satyanarayana, B. Arkivoc, 2008, 13, 122-128.
- Sebti, S.; Nazih, R.; Tahir, R.; Salhi, L.; Saber, A. Appl. Catal. A: Gen. 2000, 197, L187.
CrossRef - Sebti, S.; Tahir, R.; Nazih, R.; Saber, Boulaajan, S. Appl. Catal. A: Gen. 2002, 228, 155.
CrossRef - Reddy, B. H.; Patil, M. K.; Rao, K. N. Reddy, G. K. J. Mol. Catal. A. Chem., 2006, 258, 302.
CrossRef - Harjani, J. R. Nara S. J.; Salunkhe, M. M. Tetrahedron Lett. 2002, 43, 1127.
CrossRef - Santamarta, F.; Verdia, P.; Tojo, E. Catal. Commun. 2008, 9, 1779 and Verdia, P.; Santamarta, F.; Tojo, E.; Molecules, 2011, 16, 4379-88.
- Ren, Y.; Cai, C. Synth. Commun., 2007, 37, 2209.
CrossRef - Khan, F. A.; Dash, J.; Satapathy, R.; Upadhay, S. K. Tetrahedron Lett. 2004, 45, 3055.
CrossRef - Ethamifar, D.; Kazempoor, S.; J. Mol. Catal A; Chemical 2016, 415, 74-81.
- Panchenko, V. N.; Matrasova, M. M. Jeon, J.; Jun, J.W.; Timofeera, M. N.; Jhung, S.W. J. of Catal. 2014, 316, 251-59.
CrossRef - Peng, Y. Q.; Song, G. H. Green Chem. 2003, 6, 704.
CrossRef - Ferocis, M.; Orsini, M.; Palombi, L; Inesi, A. Green Chem. 2007, 4, 323 and Bhuiyan, M.M.H.; Hossain, M.I.; Alam, M.A.; Mahmud, M.M. Chem. J. 2012, 1, 31-37.
- Momoyezan, M.; Gheshang, M.; Hassanzadeh-Tabrizi, S.A. Bulgarian Chem. Commun. 2015, 47, 809-15.
- Zamani, F.; Izadi, E.; Chin. J. Catal. 2014, 35, 21-27.
CrossRef - Kolahdoozan, M.; Kalbesi, R. J.; Shahzeidi, Z. S.; Zamani, F. J. of Chem. doi, 10.1155/2013/49 687.
- Bouhrara, M.; Ranga, C.; Fihri, A.; Shaikh, R. R.; Sarawade, P.; Emvas, Abdul-Hamid, Hedhili, M.N. Polshettiwar, V. ACS Sustainable Chem. Engg. 2013, 1, 1192-1199.
CrossRef - Li, G.; Yiao, J.; Zhang, W. Green Chem., 2012, 14, 2234.
CrossRef - Saha, M.; Pal, A.K. Advances in Nanoparticles, 2012, 1, 61-70.
CrossRef - Subhadip, N.; Sharma, M.K., Bharadwaj, P. K. J. Mol. Catal. A: Chemical 2009, 299, 1-4.
CrossRef - Sharma, M.K.; Singh, P.P.; Bharadwaj, P.K. J. Mol. Catal. A: Chemical 2011, 342, 6-10.
CrossRef - Ren, Y.; Lu, J.; Jiang, O.; Cheng, J.; Chin. J. Catal. 2015, 36, 1949-56.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









