Antibacterial Activity of Different Extracts of Centaurea Cyanus (L.) Growing Wild in Kosovo
Arben Haziri1, Fatmir Faiku1, Ibrahim Rudhani2, Ibrahim Mehmeti3 and Diedona Motori1
1Department of Chemistry, Faculty of Natural Sciences, University of Prishtina Hasan Prishtina, George Bush Street, p.n., Kosovo.
2Faculty of Medicine, University of Prishtina Hasan Prishtina, George Bush Street, p.n., Kosovo.
3Biotechnology and Food Science, Norwegian University of Life Sciences, Ås, Norway.
Corresponding Author E-mail: ibrahim.rudhani@uni-pr.e
DOI : http://dx.doi.org/10.13005/ojc/330406
In this study the antibacterial efficiency of different organic extracts from Centaurea cyanus (L.) growing wild in Kosovo were examined. Methanol, ethyl acetate, acetone, diethyl ether, water and chloroform extracts were tested against three gram positive bacteria Staphylococcus aureus (food isolate), Staphylococus aureus (clinical isolate), Listeria monocytogenes (clinical isolate) one gram negative bacteria Escherichia coli (clinical isolate). The antibacterial activity was determined by using agar disc diffusion method. The inhibition zone of extracts was compared to that of penicillin G as standard. Based on the results, the most intense activity was shown by the plant`s extracts with water and ethyl acetate. The ethyl acetate extract showed activity in all of the concentrations 1, 3 and 5 mg/mL towards S. aureus (food isolate), E. coli (clinical isolate), S. aureus (clinical isolate) and L. Monocytogenes (clinical isolate). Ethyl acetate extract of the plant with concentration 5 mg/mL showed a stronger antibacterial activity towards bacteria E. coli with inhibition zone of 12 mm. Aqueous extract of the plant with concentration 5 mg/ mL showed a stronger antibacterial activity against bacteria E. coli with inhibition zone of 12 mm. Also aqueous extracts of the C. cyanus (L.) showed a stronger antibacterial activity as penicillin G against bacteria S.s aureus (clinical isolate). From the obtained results, the most antibacterial activity has water and ethyl acetate extracts. The antibacterial activity of the C. cyanus (L.) was due to the presence of various secondary metabolites such as phenols and flavonoids. Hence, this plant can be used to discover bioactive natural products that may serve as leads in the development of new pharmaceuticals.
KEYWORDS:Centaurea cyanus (L.); antibacterial activity; agar disk diffusion method; organic extracts
Download this article as:| Copy the following to cite this article: Haziri A, Faiku F, Rudhani I, Mehmeti I, Motori D. Antibacterial Activity of Different Extracts of Centaurea Cyanus (L.) Growing Wild in Kosovo. Orient J Chem 2017;33(4). |
| Copy the following to cite this URL: Haziri A, Faiku F, Rudhani I, Mehmeti I, Motori D. Antibacterial Activity of Different Extracts of Centaurea Cyanus (L.) Growing Wild in Kosovo. Orient J Chem 2017;33(4). Available from: http://www.orientjchem.org/?p=35389 |
Introduction
The heterogeneous relief of Kosovo with the diversity of soil types is responsible for the heterogeneousness of the Kosovo flora1. Terpenes, phenolics and nitrogen-containing compounds are responsible for the biological activities of the medicinal plants. The antibiotics drugs extracted from plants are of greater interest in comparison to synthetic ones. The use of natural antibiotics from plants does not induce the secondary health effects (abdominal pain, diarrhea, cramping, headache, nausea and vomiting etc). Plant products may be used as antibiotic alternatives and do not cause resistance in bacteria2.
C. cyanus (L.), commonly known as cornflower, is a flowering plant belonging to the family of Asteraceae. Their extracts were used in folk medicine in Kosovo for different purposes. The antibacterial effect of the extracts, obtained from the medicinal plants, has been studied by a number of researchers in different parts of the world3-11.
Our research group was interested to analyze the biological activities of different medicinal plants extracts growing in different region of Kosovo12-19. The aim of this study was to investigate the antibacterial activity of solvent extracts of C. cyanus (L.) growing wild in Kosovo.
Materials and Methods
Plant Material
The C. cyanus (L.) plant was collected in 23. 06. 2015 (Figure 1) in Kosovo. Voucher specimens were deposited in the herbarium of the Department of Biology, University of Prishtina. The aereal part of this medicinal plant were air dried at room temperature within three weeks.
 |
Figure 1: C. cyanus (L.) growing wild in Kosovo (photo taken from Arben Haziri) |
Preparation of Plant Organic Extracts
The aerial part of C. cyanus (L.) was air-dried and then milled with a mixer. A piece of finely powdered material (200 g) was extracted with 70% methanol (CH3OH, 4 L) during a 24 h period (three times). After removing the CH3OH under reduced pressure, the aqueous phase is extracted with four consecutive increasing polarity solvents, diethyl ether (C4H10O), chloroform (CHCl3), etilacetate (CH3COOCH2CH3) and acetone (CH3COCH3). Extraction is done until colorless extracts are taken. The remaining was water extract. Five extracts (C4H10O, CHCl3, CH3COOCH2CH3, CH3COCH3 and H2O) were evaporated by vacuum rotary evaporator (EYELA N-1000, Japan) to obtain crude product. The crud product then dissolved in DMSO to prepare the solution with the concentration 1, 3 and 5 mg/mL. These solutions are used in subsequent experiments for testing their antibacterial and antifungal activity. Solvents (analytical grade) for extraction were obtained from Sigma–Aldrich and Merck.
Antibacterial Activity
The antibacterial activity of the C. cyanus (L.) extracts obtained by using the different organic solvents such as methanol, ethyl acetate, acetone, diethyl ether, water and chloroform of C. cyanus (L.) are determined applying the Kirby-Bayer20 method or disk method (d = 5.5 mm, maximum capacity 10 mg). C. cyanus (L.) extracts were tested in an in vitro experiment against bacterial strains; S. aureus (food isolate with code 3321), S. aureus (clinical isolate with code 3319), L. monocytogenes (clinical isolate with code 2653) and E. coli (clinical isolate with code 2813). For the research we used three diferent concentration of extracts, 1, 3 and 5 mg/mL in DMSO as solvent and then placed in a Petri dishes (d=15 cm). The disks were incubated at 370C for 48 h; the control was also maintained with penicillin G dissolved in DMSO in a similar manner.
Results and Discussion
During the antibacterial activity, we have tested the extracts from the plant C. cyanus (L.) against four bacteria: S. aureus (positive Gram bacteria-food isolate), L. monocytogenes (positive Gram bacteria-clinical isolate), E. coli (negative Gram bacteria-clinical isolate) and S. aureus (positive Gram bacteria-clinical isolate). Antibacterial activity of these extracts were compared with Penicillin G- a substance, which can be found in the market and have been used with concentration 1, 3 and 5 mg/ mL as a standard. The comparison is done in accordance to the measurements of the diameter of the inhibition zone that is formed. These results are shown in table 1.
Table 1: Inhibition zones diameters of Centaurea cyanus (L.) extract
| Extracts | Concentration(mg/ml) | Inhibition zones diameters (mm) | |||
| E. coli (c. i.) | L. monocytogenes(c. i.) | S. aureus(f. i.) | S. aureus(c. i.) | ||
| Methanol | 135 | 677 | –5 | 668 | –5 |
| Ethyl acetate | 135 | 4612 | 669 | 3610 | 447 |
| Acetone | 135 | –5 | –4 | 457 | –4 |
| Diethyl ether | 135 | -56 | –5 | –5 | 457 |
| Water | 135 | 7912 | -67 | -710 | 7811 |
| Chloroform | 135 | 677 | –4 | 457 | -34 |
| Penicillin | 135 | 468 | 268 | 4810 | 2610 |
(-) no inhibition zone
Extracts of methanol (1, 3 and 5 mg/ mL), ethyl acetate (1, 3 and 5 mg/mL), acetone (5 mg/mL), diethyl ether (3 and 5 mg/mL), chloroform (1, 3 and 5 mg/mL) and water with concentration 1, 3 and 5 mg/mL shows antibacterial activities against E. coli (Table 1 and Figure 2).
The extracts of methanol, acetone, chloroform and diethyl ether with concentration of 5 mg/mL resulted in a lower activity than the penicillin G of the same concentration. The extract of ethyl acetate with concentration of 3 mg/mL and 5 mg/mL has the same inhibition zone as the standard (6 mm). Extracts of methanol (1 and 3 mg/mL), ethyl acetate (5 mg/mL) water (1, 3 and 5 mg/mL) and chloroform (1 and 3 mg/mL) created an inhibition zone higher than the penicillin G with the same concentrations. The extract of chloroform with concentration 5 mg/mL resulted in lower activity than the penicillin G with the same concentration. The other extracts such as acetone (1 and 3 mg/mL) and diethyl ether with concentration 1 mg/mL do not create any inhibition zone, in other words they do not show activity (Table 1 and Figure 2).
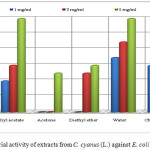 |
Figure 2: Antibacterial activity of extracts from C. cyanus (L.) against E. coli (clinical isolate). |
The extract of ethyl acetate with concentration of 1 and 5 mg/mL (inhibition zone 6 mm respectively 9 mm) resulted in a higher activity against L. monocytogenes than the penicillin G (inhibition zone 2 mm respectively 8 mm). The extracts of ethyl acetate and water with concentration of 3 mg/mL have the same inhibition zone as the standard (inhibition zone 6 mm). The extracts of methanol, acetone, diethyl eter chloroform and water with concentration of 5 mg/mL resulted in a lower activity than the penicillin G of the same concentration with inhibition zone of 8 mm. The other extracts such as methanol (1 and 3 mg/mL), acetone (1 and 3 mg/mL), diethyl ether (1 and 3 mg/mL) chloroform (1 and 3 mg/mL) and water with concentration 1mg/mL did not show any inhibition activity on bacteria L. monocytogenes as was shown in Table 1 and Figure 3.
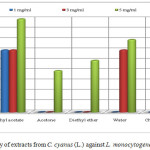 |
Figure 3: Antibacterial activity of extracts from C. cyanus (L.) against L. monocytogenes (clinical isolate) Click here to View figure |
The extracts of methanol, ethyl acetate, acetone and chloroform showed antibacterial activity against S. aureus (isolated in food) in all of the concentrations 1, 3 and 5 mg/mL. The extracts of ethyl acetate (5 mg/mL), water (5 mg/mL), acetone(1 mg/mL) and chloroform with concentration of 1 mg/mL has the same inhibition zone. The extract of methanol with concentration of 1 mg/mL (inhibition zone 6 mm) resulted in a higher activity against S aureus (isolated in food) than the penicillin G (inhibition zone 4 mm). The extracts of methanol (6 mm), ethyl acetate (6 mm), acetone (5 mm), water (7 mm) and chloroform (5 mm) with concentration of 3 mg/mL resulted in a lower activity than the standard of the same concentration with inhibition zone of 8 mm. The extracts of methanol (8 mm), acetone (7 mm), diethyl eter (5 mm) and chloroform (7 mm) with concentration of 5 mg/mL resulted in a lower activity than the standard of the same concentration with inhibition zone of 10 mm. The extracts of diethyl ether (1 and 3 mg/mL and water with concentration 1 mg/mL did not show activity on bacteria S. aureus isolated in food (Table 1 and Figure 4).
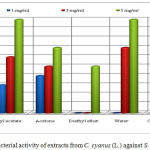 |
Figure 4: Antibacterial activity of extracts from C. cyanus (L.) against S. aureus (food isolate) Click here to View figure |
The extracts of ethyl acetate (4 mm), chloroform (3 mm) and diethyl ether (5 mm) with concentration of 3 mg/mL resulted in a lower activity against S. aureus (positive Gram bacteria) isolated in the clinical way, than the standard with the same concentration (inhibition zone of 6 mm). The extract of water with concentration of 1 and 3 mg/mL (inhibition zone 7 mm respectively 8 mm) resulted in a higher activity than the penicillin G (inhibition zone 2 mm respectively 6 mm). The extracts of methanol, ethyl acetate, acetone, diethyl ether and chloroform with concentration of 5 mg/mL resulted in a lower activity than the penicillin G of the same concentration (inhibition zone10 mm). Aqueous extract of the showed a stronger antibacterial activity to bacteria S. aureus isolated in the clinical way.
The other extracts such as methanol (1 and 3 mg/mL), acetone (1 and 3 mg/mL) and chloroform with concentration 1 mg/mL do not create any inhibition zone, in other words they do not show activity (Table 1 and Figure 5).
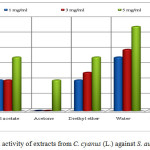 |
Figure 5: Antibacterial activity of extracts from C. cyanus (L.) against S. aureus (clinical isolate) |
Conclusion
C. cyanus (L.) is a medicinal plant used in folk medicine for different health illnes. The aim of this research was to analyse the antibacterial activities of different extracts from C. cyanus (L.) plant growing wild in Kosovo. Based on the results, the most intense activity was shown by the plant`s water and ethylacetate extracts. The three concentrations of the extract with ethyl acetate showed a very good activity by inhibiting the growth of all the tested bacteria. Ethyl acetate extract of the plant with concentration 5 mg/mL showed a stronger antibacterial activity towards bacteria S. aureus (food isolate), E. coli (clinical isolate), S. aureus (clinical isolate) and L.monocytogenes (clinical isolate). Water and ethyl acetate extracts with concentration 5 mg/mL showed a stronger antibacterial activity (inhibition zone 12 mm) against bacteria E. coli. Thus, these extracts showed higher activity than all other extracts. Results obtained from water and ethyl acetate extracts for antibacterial activity are logical, based on numerous of studies where these extracts were analyzed in the content of flavonoids, phenols, terpenes, alkaloids, etc., and these components are responsible for the biological activity.
Acknowledgements
We would like to thank Prof. Dr. Elez Krasniqi, from the Department of Biology at University of Prishtina, for identifying the plant Centaurea cyanus (L.).
References
- Mehmeti, A.; Sherifi, E.; Demaj, A. Medical Herbs (European Agency for Reconstruction), First edition, Prishtinë (Kosovo). 2007, 46-47.
- Gibbons, S. Phytochemistry Rev. 2005, 4, 63-78.
CrossRef - Nurettin, Y.; Ahmet, Y.; Canan, G.; Asu, U.; Sevgi, K.; Kamil, C.; Şengül, K. Phytochemistry, 2005, 66, 1741–1745.
CrossRef - Uysal, I.; Celik, S.; Oldacay, M. Pakistan J. Biological. Sci. 2005, 8, 1812-1813.
CrossRef - Shoeb, M.; Stephen, M.; Mac, M.; Marcel, J.; Paul, K. T. L.; Lutfun, N.; Sezgin, C.; Satyajit, D. S. Rev. Bras. Farmacogn. 2007, 17, 155-159.
CrossRef - Guiamet, P. S.; De la Paz Naranjo, J.; Arenas, P. M.; Gómez de Saravia, S. G. Pharmacologyonline, 2008, 3, 649-658.
- Stanojković, A.; Ceković, J.; Čomić, L.; Pivić, R.; Stanojković, A. Lek. Sirov. 2008, 26/27, 11-20.
- Baharfar, R.; Mohammad, A. K.; Siamak, G.; Omid, J.; Razieh, A.; Mahmood, T. Iran. J. O. C. 2009, 3, 172-177.
- Cansaran, A.; Mercan, N.; Öztekin, M.; Gülümser, A. Afr. J. Microbiol. Res. 2010, 4, 608-612.
- Tekeli, Y.; Gokhan, Z.; Abdurrahman, A.; Mehmet, S.; Emrah, T. Arch. Biol. Sci. 2011, 63, 685-690.
CrossRef - Astari, K. A.; Şura, B. E.; Kose, F. A.; Koksal, E.; Karaalp, P. Turkish J. Pharm. Sci. 2014, 11, 101-106.
- Faiku, F.; Haziri, A.; Mehmeti, I.; Bajrami, D.; Haziri I. The J. Anim.Plant Sci. 2016, 26, 1486-1491.
- Faiku, F.; Haziri, A.; Gashi, F.; Troni, N.; Faiku, H. Eur. Chem. Bull. 2015, 4, 331-334.
- Haziri, A.; Faiku, F.; Mehmeti, A.; Govori, S.; Abazi, S.; Daci, M.; Haziri, I.; Bytyqi-Damoni, A.; Mele, A. Am. J. Pharmacol. Toxicol. 2013, 8, 128-133.
CrossRef - Faiku, F.; Haziri, A. Eur. Chem. Bull. 2015, 4, 432-435.
- Faiku, F.; Haziri, A. Int. J. Pharm. Phytopharmacol. Res. 2013, 3, 254-257.
- Faiku, F.; Haziri, A.; Domozeti, B.; Mehmeti, A. Euro. J. Exp. Bio. 2012, 2, 1273-1277.
- Haziri, A.; Aliaga, N.; Ismaili, M.; Govori, S.; Leci, O.; Faiku, F.; Arapi, V.; Haziri, I. Am. J. Biochem. Biotechnol. 2010, 6, 32-34.
CrossRef - Haziri, A.; Govori, S.; Ismaili, M.; Faiku, F.; Haziri, I. Am. J. Biochem. Biotechnol. 2009, 5, 226-228.
CrossRef - Barry, L. Procedure and Theoretical Consideration for Testing Antimicrobial Agents in Agar Media. 5th edition William Wilkins Baltimore, 1991, 8-10.

This work is licensed under a Creative Commons Attribution 4.0 International License.









