Technology Basis and Thermodynamic Analysis of an Acid Decomposition Process of Phosphorus Slime
Ulzhalgas Nazarbek1, Uilesbek Besterekov1, Saule Nazarbekova2, Aidar Bolysbek1 and Galya Kambarova1
1Department of “Chemical technology of inorganic substances”.
2Department of “Chemistry”, M. Auezov South Kazakhstan State University, 5 Tauke-khan ave., Shymkent, 160000, Kazakhstan.
Corresponding Author E-mail: unazarbek@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/330311
The given article contains the research results connected with the development of a technology of acid decomposition of phosphorus slime. As opposed to a known technology of manufacturing superphosphate, we have applied a double superphosphate production technology as a basis. Slime raw material is fed to both stages of acid decomposition; the first stage is sulphuric acid decomposition, and the second stage – phosphoric acid one. The basic chemistry of two step processing of phosphorus slime is described. Thermodynamic analyzes of the described reactions are given. According to the thermodynamic analysis the Law’s of phosphorus slime decomposition were found. As a result the end product after phosphoric-acid decomposition contains basically Са(Н2РО4)2, an insignificant quantity of СаНРО4, water-soluble magnesium and potassium phosphates and also the insoluble residue (SiO2). According to the thermodynamic analysis, the decomposition regularities of phosphorus slime were revealed. The ΔG0 values of sulfuric and phosphoric acids decomposition of phosphorus slime at the temperature of 600°C were compared.
KEYWORDS:Phosphoric slime; sulphuric and phosphoric acids; processing; superphosphate; thermodynamic analysis
Download this article as:| Copy the following to cite this article: Nazarbek U, Besterekov U, Nazarbekova S, Bolysbek A, Kambarova G. Technology Basis and Thermodynamic Analysis of an Acid Decomposition Process of Phosphorus Slime. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Nazarbek U, Besterekov U, Nazarbekova S, Bolysbek A, Kambarova G. Technology Basis and Thermodynamic Analysis of an Acid Decomposition Process of Phosphorus Slime. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33662 |
Introduction
The given article contains the research results connected with the development of a technology of acid decomposition of phosphorus slime. As opposed to a known technology of manufacturing superphosphate1-2, we have applied a double superphosphate production technology as a basis. Slime raw material is fed to both stages of acid decomposition; the first stage is sulphuric acid decomposition, and the second stage – phosphoric acid one.
The process diagram is represented in figure 1.
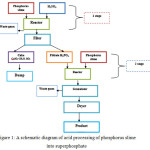 |
Figure 1: A schematic diagram of acid processing of phosphorus slime into superphosphate
|
Materials and Methods
Chemism of the first stage of the decomposition process (obtaining phosphoric acid) of phosphorus slime is described by known reactions:
Phosphate
Ca5(PO4)3F + 5H2SO4 + 10H2O = 3H3PO4 + 5CaSO4 · 2H2O + HF (1)
Impurities
СаСО3 + H2SO4 + H2O = CaSO4 · 2H2O + СО2. (2)
MgСО3+ H2SO4 + H2O =MgSO4 + СО2+ 2H2O. (3)
K2O + H2SO4 = К2SO4 + H2O. (4)
The researches have shown, that main products of the first stage of sulphuric acid decomposition of phosphorus slime are a phosphoric acid solution with concentration of 14-17 % of Р2О5 and a cake – the sediment containing mainly calcium sulphate and insoluble impurities (SiO2). Thus, in the soft dihydrate regime (60оС, formation of calcium sulphate dihydrate) a weak phosphoric acid is formed, which is fed to the second (phosphoric acid) stage of decomposition of a new portion of phosphorus slime for the purpose of obtaining a calcium-phosphate product according to the reactions:
Phosphate
Ca5(PO4)3F + 7H3РO4 + 5H2O = 5Ca(H2PO4)2 · H2O + HF. (5)
Impurities
СаСО3 + 2H3РO4 = Ca(Н2РO4)2·H2O + СО2. (6)
MgСО3+ 2H3РO4 = Mg (Н2РO4)2 · H2O + СО2. (7)
K2O + H3РO4 = К2НРO4 + H2O. (8)
At this stage potassium with phosphoric acid forms К2НРО4, which by entering in the exchange interaction with Са(Н2РО4)2 can turn into a more acid salt – КН2РО4, and the phosphate anion liberated reacts with a calcium ion and forms almost insoluble СаНРО4 in accordance with the reaction:
К2НРO4 + Са(Н2РО4)2 = 2КН2РO4 + СаНРО4. (9)
As a result the end product after phosphoric-acid decomposition contains basically Са(Н2РО4)2, an insignificant quantity of СаНРО4, water-soluble magnesium and potassium phosphates and also the insoluble residue (SiO2)3-4.
Results
A thermodynamic analysis of the reactions proceeding at the acid decomposition of phosphorus slime with impurities of carbonate, acidic and alkaline compounds has been carried out with taking into account phase changes of initial components and end-products and calculation of enthalpy, entropy and Gibbs energy changes and an equilibrium constant logarithm. The calculations have been fulfilled using a multifunctional software package HSC-5.1. This software program is widely applied in European universities; it has been developed by a Finnish metallurgical company Outokumpu. The program database contains information about more than 17000 substances5.
The process thermodynamic parameters have been determined in a temperature interval of 290-353К. The calculated thermodynamic characteristics depending on temperature and foreign component composition for the guess reactions of synthesis of phosphorus- and potassium-containing mineral fertilizers are represented in figures 2-12.
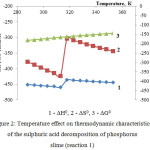 |
Figure 2: Temperature effect on thermodynamic characteristics of the sulphuric acid decomposition of phosphorus slime (reaction 1)
|
Two groups of chemical reactions have been studied: the first group includes the reactions of phosphoric acid and impurities formation (reactions 1-4); the second group – formation of monocalcium phosphate and impurities (reaction 5-9).
The data (fig.2) are evidence of thermodynamic feasibility of the sulphuric acid decomposition of phosphorus slime in the investigated temperature area according to the reaction 1. The enthalpy and entropy jumps at 313К are caused by the phase changes which are taking place in the considered system.
For establishment of the jump (change) nature for dependences ∆Н0 =f(T) and ∆S0 =f(T) we have determined temperature influence on ∆Н0 and ∆S0 of all the reacting substances of reaction 1 according to figure 3.
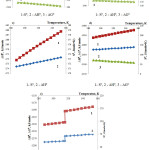 |
Figure 3: Temperature effect on thermodynamic characteristics of the substances in the reaction 1
|
As follows from the figure 3, the observed change on the curves is connected with phosphoric acid properties. In this case there are first-type phase transitions.
The figures 4 and 5 represent the thermodynamic research results of decomposition of calcium and magnesium carbonates by sulfuric acid according to the reactions 2 and 3.
Apparently the changes of thermodynamic parameters of sulphuric acid decomposition of impurity calcium and magnesium carbonates contained in the phosphorus slime have identical nature. Values of ∆G0 in the investigated temperature range are in a negative area; it proves thermodynamic probability of the reactions 2-3.
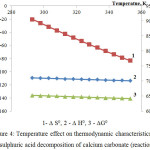 |
Figure 4: Temperature effect on thermodynamic characteristics of the sulphuric acid decomposition of calcium carbonate (reaction 2)
|
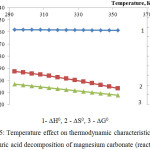 |
Figure 5: Temperature effect on thermodynamic characteristics of the sulphuric acid decomposition of magnesium carbonate (reaction 3)
|
The thermodynamic research results of potassium oxide sulphuric-acid decomposition in accordance with the reactions 4 are represented in fig.6.
As follows from this figure, Gibbs energy in the considered temperature interval has negative values, and it testifies to thermodynamic probability of the reaction 46.
Judging by comparison of the ∆G0 values for the first reaction group we can say that from the thermodynamic point of view the probability of these reactions (at temperature 333К) changes in the following sequence: K2O ˃ Ca5(PO4)3F ˃ СаСО3 ˃ MgСО3.
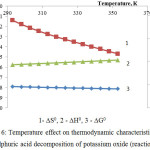 |
Figure 6: Temperature effect on thermodynamic characteristics of the sulphuric acid decomposition of potassium oxide (reaction 4) |
At the same time all the reactions of the given group are exothermal because of corresponding ∆Н0 have negative values.
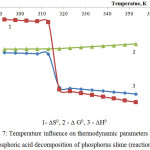 |
Figure 7: Temperature influence on thermodynamic parameters of the phosphoric acid decomposition of phosphorus slime (reaction 5) |
A dependence of thermodynamic parameters of the phosphoric acid decomposition of phosphorus slime (in accordance with the reaction 5) on temperature is represented in fig.7.
The results confirm thermodynamic probability of the reaction of phosphoric acid decomposition of the phosphate part of phosphorus slime in the investigated temperature area.
The observed jumps of enthalpy and entropy at 313К are caused by the first-type phase change, which occur in the phosphoric acid structure.
Figures 8 and 9 represent results of the thermodynamic research of calcium and magnesium carbonates decomposition by phosphoric acid (reactions 6 and 7).
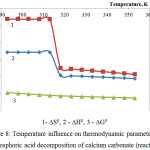 |
Figure 8: Temperature influence on thermodynamic parameters of the phosphoric acid decomposition of calcium carbonate (reaction 6) |
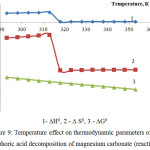 |
Figure 9: Temperature effect on thermodynamic parameters of the phosphoric acid decomposition of magnesium carbonate (reaction 7)
|
As follows from the figures 8 and 9, the changes of thermodynamic parameters of processes of phosphoric acid decomposition of foreign components contained in the phosphorus slime – calcium and magnesium carbonates – have the same nature. Values of ∆G0 for the reactions 6-7 in the investigated temperature range are in a negative area; it proves thermodynamic probability of the given reactions.
Results of the thermodynamic research of the processes proceeding according to the reactions 8 and 9 are represented in fig.10-11.
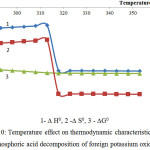 |
Figure 10: Temperature effect on thermodynamic characteristics of the phosphoric acid decomposition of foreign potassium oxide
|
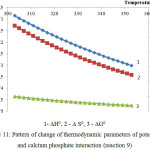 |
Figure 11: Pattern of change of thermodynamic parameters of potassium and calcium phosphate interaction (reaction 9) |
As follows from the data of the figures 10 and 11, the reactions 8 and 9 in the considered temperature conditions are realizable7.
Proceeding from the received ∆G0 values it is possible to conclude, that the basic substances of the second reaction group react with phosphoric acid in the following sequence: K2O ˃ MgСО3 ˃ Ca5(PO4)3F ˃ СаСО3.
Thus, it has been established, that from the thermodynamic point of view the reactions 1 and 5 of acid decomposition of the phosphate part of phosphorus slime and the reactions 2, 3, 4, 6, 7, 8 and 9 describing the impurity components decomposition are probable.
References
- Pozin M.E. Technology of mineral fertilizers: a textbook for institutions of higher education. – 6 ed., revised. – Leningrad: Chemistry, 1989, 352.
- Nazarbek U.B., Besterekov U.B., Petropavlovsky I.A., Nazarbekova S.P., Pochitalkina I.A., European Conference on Innovations in Technical and Natural Sciences. Vienna, 2015, 114-123.
- Kopylyov B.A. Wet-process phosphoric acid technology. – Leningrad: Chemistry, 1981, 224.
- Kopylov V.A. Manufacture of double superphosphate. – Мoscow: Chemistry, 1976, 192.
- Roine A. Outokumpu HSC Chemistry for windows Chemical reaction and Equilibrium soft with extensive thermo chemical data base. – NY, 2002 //http://www.outotec.com.
- Besterekov U.B. Theoretical foundation of inorganic substance technology. Shymkent,, 2014, 178.
- Alekseyev A.I., Kulinich O.V., Ramzanova L.P., Yuzvyak S. Thermodynamic reaction analysis in chemical technology: a study guide. – St.Petersburg, 2003, 135.

This work is licensed under a Creative Commons Attribution 4.0 International License.









