Synthesis, Spectroscopic Characterization and Pharmacological Evaluation of Schiff Base molecular Adducts of Copper Metal
Richa Kothari
Department of Chemistry, ITM University Gwalior India 474011.
Corresponding Author E-mail: Richakothari70@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330340
A series of copper complexes were prepared from N4 – substituted Schiff base ligands having structure [Cu (L)2] Cl2 ; where L= p-methoxybenzylidene ethylenediammine, p-nitrobenzylidene ethylenediammine, p- chloroacetophenone ethylenediammine, p-methoxy acetophenone ethylenediammine, p-nitro acetophenone ethylenediammine. All copper complexes were characterized by various physico-chemical techniques like melting point,TLC, elemental analysis,IR,H1-NMR,LC-MS,UV-Visible spectra. The magnetic moments and electronic spectral studies suggested distorted octahedral geometry for all the complexes. The monoanionic ethylene diammine ligands act in a bi dentated mode, binding through azomethine nitrogen atoms.. The synthesized compounds were screened for their in vitro antibacterial activity using Disc Diffusion method against two strains of gram negative and gram positive bacteria. Tetracycline antibiotic was used as positive control in this test. All the compounds showed significant antibacterial activity in the range of 1-10 mg/ml. Antioxidant activity of the all copper complexes was screened using the DPPH scavenging assay. In this activity ascorbic acid was used as a positive control .All complexes exhibited potent antioxidant activity in the range 50-75%. These compounds would be evaluated further for their possible DNA binding, cleavage, antifungal and anti-diabetic activities.
KEYWORDS:Copper (II) complexes; schiff bases; molecular adducts; pharmacological evaluation Invitro-antibacterial; antioxidant activity
Download this article as:| Copy the following to cite this article: Kothari R. Synthesis, Spectroscopic Characterization and Pharmacological Evaluation of Schiff Base molecular Adducts of Copper Metal. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Kothari R. Synthesis, Spectroscopic Characterization and Pharmacological Evaluation of Schiff Base molecular Adducts of Copper Metal. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33190 |
Introduction
Metal-based antitumor drugs play a relevant role in antiblastic chemotherapy. Cisplatin is regarded as one of the most effective drugs, even if severe toxicities and drug resistance phenomena limit its clinical use. Therefore, in recent years there has been a rapid expansion in research and development of novel metal-based anticancer drugs to improve clinical effectiveness, to reduce general toxicity and to broaden the spectrum of activity. The variety of metal ion functions in biology has stimulated the development of new metallodrugs other than Pt drugs with the aim to obtain compounds acting via alternative mechanisms of action. Among non-Pt compounds, copper complexes are potentially attractive as anticancer agents. Actually, since many years a lot of researches have actively investigated copper compounds based on the assumption proposal that endogenous metals may be less toxic. It has been established that the properties of copper-coordinated compounds are largely determined by the nature of ligands and donor atoms bound to the metal ion. Organo-metallic compounds have been used in medicine for centuries. Metal play essential role in pharmaceutical industry. The metallo elements present in trace quantities play vital role at the molecular level in the system. Copper as a component of numerous enzymes is involved in energy production, is necessary for neurotransmission in the brain and is active in cell protection from the damage generated by the free radicals. The copper deficiency is associated with the anemia and bone demineralization. Copper is most abundant and essential metal in our body system. Cu(II)- based complexes .Cu(II)- based complexes appear to very promising candidates for anticancer therapy, an idea supported by a considerable number of researchers [2-5] describing the synthesis and cytotoxic activities of numerous Cu(II) complexes and copper (II) complexes containing Schiff bases have also displayed biological properties [6-8]. Schiff base macrocyclic ligands based on ethylenediammine and its metal complexes have received considerable attention. Because of their pharmacological properties, they have numerous applications as antibacterial and anticancer agents [9-11].They can yield mono or polynuclear complexes, some of which are biologically relevant [12-15]. Copper complexes can serve as models for enzymes such as galactose oxidase and may be used as effective oxidants and redox catalysts [16-17]. Furthermore, they allow extraction of metallic cations and anions of biochemical and environmental importance [18-21]. Macrocyclic ligand complexes find applications in various industries and in anumber of biological processes such as photosynthesis and dioxygen transport [22], catalysis, metal extractants, radiotherapy, medical imaging agents, DNA binding [23] and antitumor agents. It has been known for many years that a large number of Schiff base ligands and series of their copper complexes have promising antitumor activities [24-26]. A critical property of many copper (II) complexes is their poor water solubility and their relatively high in vivo toxicity [27-28]. Many attempts have been made to improve hydrophilicity and reduce toxicity by modifying the thiosemicarbazone framework [29]. In recent years, several series of copper complexes have been studied as potential antitumor agents. Although scanty information is available of the molecular basis of their mechanism of action, copper complexes have attracted attention because their probable mode of action is different from that of cisplatin. Therefore, copper complexes may provide a broader spectrum of antitumor activity. Elemental analysis and spectroscopic analysis suggested the distorted octahedral geometry for these complexes.
In this paper, the most remarkable achievements in the design and development of copper (II) complexes as antitumor agents are discussed. Special emphasis has been focused on the identification of structure-activity relationships for the different classes of copper (I, II) complexes. This work was motivated by the observation that no comprehensive surveys of copper complexes as anticancer agents were available in the literature. Moreover, up to now, despite the enormous efforts in synthesizing different classes of copper complexes, very few data concerning the molecular basis of the mechanisms underlying their antitumor activity are available. This overview, collecting the most significant strategies adopted in the last ten years to design promising anticancer copper(I,II) compounds, would be a help to the researchers working in this field.
Materials and Methods
Chemistry
All glass wares were dried in an open flame before use in connection with an inert atmosphere. Solvents were evaporated under reduced pressure and evaporation was carried out at <50°C. TLC was performed using silica gel60F254 plates with iodine vapors as detecting agent. Tetra methyl silane (TMS; 0.0 ppm) was used as an internal standard in 1HNMR and Chloroform-d (CDCl3; 77.0 ppm) was used in 13C NMR. Elemental analysis was carried out on a Perkin Elmer 2400 series 11 CHNS/O elemental analyzer. FTIR spectra were recorded using KBr pellets on Perkin Elmer-Spectrum RX-IFTIR in the 4000-250 cm-1 region. The electronic spectra in DMSO solution were obtained with a Hitachi 330 uv-vis-nir spectrophotometer. The FAB-Masses in positive mode were recorded on a Waters Micromass Q-Tof spectrometer; m-Nitro benzyl alcohol (m-NOBA) was used as the matrix. Melting points were determined by open capillary method. All materials were obtained from commercial suppliers such as Merck, CDH, SRL and were used without further purification. The solvents and copper salts used were of analytical grade. Various hydrazones of complexation agent were prepared by standard methods described in the literature [30].
Synthesis of Schiff base molecular adducts of copper metal
A mixture of hydrated copper chloride, compound (1-5) and ethylenediammine hydrochloride in 2:2:1 molar ratio in absolute ethanol were added slowly with stirring in a round bottom flaskat 70-800c for 8 hours. Solvent was evaporated under reduced pressure and the residue obtained was quenched with ethanol. The solid product was precipitated, filtered off, washed several times with cold ethanol and dried over fused CaCl2 in desiccators. A good yield of product was obtained and the purity of the complex was confirmed by the TLC and the elemental analysis.
Where R1and R2 substitutions are as follows
| S.No. | Compound | R1 | R2 |
| 1. |
[Cu (p-macehen)2 ]Cl2 |
-CH3 | -OCH3 |
| 2. |
[Cu (p-nacehen)2 ]Cl2 |
-CH3 | -NO2 |
| 3. |
[Cu (p-clbhen)2] Cl2 |
-H | -Cl |
| 4. |
[Cu (pmbhen)2 ]Cl2 |
-H | -OCH3 |
| 5. |
[Cu (p-nbhen)2]Cl2 |
-H | -NO2 |
A. [Cu (p-macehen)2 ]Cl2; bis (p-methoxyacetophenone ethylene diammine) copper II chloride
Yield: 50 %; mp 225°C; IR (KBr) (cm-1): 3299, 3034, 1581, 530; 1H-NMR (TMS) (ppm): 2.56, 2.67-2.68, 3.96-4.02 and 6.7-6.96; ESI MS: 861.5 (observed peak) other peak, 791.2, 730, 222.1, 154.1 a.m.u.; Anal. Calcd for [Cu(C42H50N10O2)]Cl2 ; C = 58.56; H = 5.81; N = 16.26. Found: C = 57.84; H = 5.97; N = 15.76; λ max = 365; Molar conductance = 46.5ohm-1cm2mho-1
B. [Cu (p-nacehen)2 ]Cl2; bis (p-nitroacetophenone ethylene diammine) copper II chloride
Yield: 76 %; mp 240 °C; IR (KBr) (cm-1): 3231, 3034, 1581, 534; 1H-NMR (TMS) (ppm): 1.1, 2.1-2.25, 3.60-3.67, 7.35-8.03; ESI-MS: 891.5 (molecular ion peak) other peak 821.3, 510.3, 400.7, 300.3, 103.2 a.m.u; Anal. Calcd for [Cu(C40H44N12O4)]Cl2; C = 53.89; H = 4.94; N = 18.86. Found: C = 53.56; H = 4.73; N = 17.51; λ max = 390; Molar conductance = 38.25ohm-1cm2mho-1
C.[Cu (p-clbhen)2] Cl2; bis (p-chlorobenzylidene ethylene diammine) copper II chloride
Yield: 40 %; mp 310 °C; IR (KBr) (cm-1): 3299, 3038, 1621, 494; 1H-NMR (TMS) (ppm): 2.1, 2.23-2.34, 3.11-3.12 and 6.81-7.9; ESI-MS: 846.3 (molecular ion peak) other peak 796.1, 490.1, 283.2, 103.0 a.m.u; Anal. Calcd for [Cu(C38H40N10Cl2)]Cl2; C = 54.18; H = 4.75; N = 16.63. Found: C = 54.21; H = 4.26; N = 16.79. λ max = 386; Molar conductance = 53.35ohm-1cm2mho-1
D. Cu (pmbhen)2 ]Cl2; bis (p-methoxybenzylidene ethylene diammine) copper II chloride
Yield: 60 %; mp 165 °C; IR (KBr) (cm-1): 3284, 2965, 1607, 522; 1H-NMR (TMS) (ppm): 1.8, 2.48-2.50, 3.42 and 7.01-7.36; ESI-MS: 831.1 (Molecular ion peak) other peak 665.1, 405.0, 192.1 a.m.u.; Anal. Calcd for [Cu(C40H46N10O2)]Cl2; C = 57.64; H = 5.52; N = 16.81. Found: C = 57.12; H = 5.89; N = 16.82; λ max = 395; Molar conductance = 23.15ohm-1cm2mho-1
F.Cu (p-nbhen)2]Cl2; bis (p-nitrobenzylidene ethylene diammine) copper II chloride
Yield: 48 % mp 230 °C; IR (KBr) (cm-1): 3345, 3030, 1597, 530; 1H-NMR (TMS) (ppm): 2.15, 2.51, 3.18-3.5 and 7.45 – 8.11; ESI-MS: 732.1 (Molecular ion peak) 551.1, 378.1, 219.1 a.m.u.; Anal. Calcd for [Cu(C38H40N12O4)]Cl2; C = 52.86; H = 4.63; N = 19.47. Found: C = 51.78; H = 4.77; N = 19.37; λ max = 410; Molar conductance = 26.25ohm-1cm2mho-1
In-vitro Antibacterial Activity
The in vitro antibacterial effects of copper complexes were evaluated against two sp. of Gram-positive bacteria (Staphylococcus aureus (MTCC 3160) and B. Subtilis (MTCC 1134)) and two Gram-negative bacteria (Escherichia coli (MTCC 50), Pseudomonas aeruginosa (MTCC 1034) by the disc diffusion method using nutrient agar medium. The bacteria were sub-cultured in the agar medium and were incubated for 24 h at 37 °C. The discs having a diameter of 5 mm, were then soaked in the test solutions (Sterile filter paper discs, What man No. 1.0) with the equivalent amount of semicarbazide Cu (II) complexes dissolved in sterile dimethyl sulphoxide (DMSO) at concentrations of 10 mg/mL and were placed in petri dishes on an appropriate medium previously seeded with microbial organisms and stored in an incubator for 24 hrs. The inhibition zone around each disc was measured and the results were recorded in the form of inhibition zones (diameter, mm). To clarify any effect of DMSO on the biological screening, separate studies were carried out using DMSO as negative control and it showed no activity against any bacterial strains. Tetracycline was used as a positive control in this antibacterial analysis.
In-vitro Antioxidant Activity
The free radical scavenging activity (RSA) of copper complexes at concentration 200, 400,600, 800, 1000 µg/ml was carried out in presence of freshly prepared solution of stable free radical DPPH (0.04% w/v) following Hataro’s method using ascorbic acid as standard. All the test analysis was performed on three triplicates and results are averaged. The results in percentage are expressed as the ratio of absorption decrease of DPPH in the presence of test compounds and absorption of DPPH in the absence of test compounds at 517 nm by UV Visible spectrophotometer. The percentage scavenging activity of the DPPH free radical was measured using following equation-
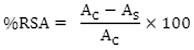
Where, AC = Absorbance of control. AS = Absorbance of test Sample
In-vitro Antineoplastic Activity
The monolayer cell culture was trypsinized and the cell count was adjusted to 1.0 x 105 cells/ml using DMEM containing 10% FBS. To each well of the 96 well microtitre plate, 0.1 ml of the diluted cell suspension (approximately 10,000 cells) was added. After 24 h, when a partial monolayer was formed, the supernatant was flicked off, washed the monolayer once with medium and 100 ml of different test concentrations of test drugs were added on to the partial monolayer in microtitre plates. The plates were then incubated at 37o C for 3 days in 5% CO2 atmosphere, and microscopic examination was carried out and observations were noted every 24 h interval. After 72 h, the drug solutions in the wells were discarded and 50 ml of MTT in PBS was added to each well. The plates were gently shaken and incubated for 3 h at 37o C in 5% CO2 atmosphere. The supernatant was removed and 100 ml of propanol was added and the plates were gently shaken to solubilize the formed formazan. The absorbance was measured using a microplate reader at a wavelength of 540 nm. The percentage growth inhibition was calculated using the following formula and concentration of test drug needed to inhibit cell growth by 50% (CTC50) values is generated from the dose-response curves for each cell line [119].
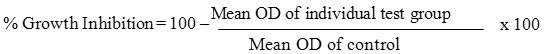
The Human Breast Carcinoma Cell Line; MCF-7 Cells were obtained from the National Center for Cell Science (NCCS), Pune India. Cells were cultured in DMEM supplemented with 10% FBS, 100U/l Penicillin, 200mg/l Streptomycin & 50mg/l Gentamicin maintained at 37°C in a humidified 5% CO2 Incubator. For experiments, cells were trypsinized and cultured in 6-well (0.2 x 106 cells/well) and 96-well (1.0 x 104/well) plates initially for 48 h so as to allow the cells to attach. After 48 h, the cells were exposed to various concentrations of complexes for the next 48 h. Each dose was tested in at least triplicate wells.
Result and Discussion
All copper complexes were synthesized by the template method. Carbohydrazone, ethylene diammine hydrochloride and CuCl2.2H2O were taken in 2:2:1 molar ratio in round bottom flask. All the complexes are stable to the atmosphere and had high melting points. Elemental analysis; C, H and N of the complexes were evaluated from SAIF, Punjab University, Chandigarh and the low molar conductance values of all the complexes in DMSO at room temperature indicated them to be non electrolytic in nature. All complexes are completely soluble in DMF, DMSO and ethanol but insoluble in water. The copper content was determined by the EDTA titration method and the nitrogen content of the complexes was determined by Kjeldahl’s method.
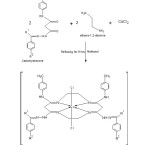 |
Figure 1: Reaction scheme of schiff base molecular adducts of Copper Click here to View figure |
LC – MS Study
Transition metal complexes were recorded under liquid secondary ion mass specral condition. All the spectra exhibited parent ion peaks due to molecular ions [M] +.
Compound (A) exhibits molecular ion peak at 861.5 that are corresponding to molecular ion peak and it gives a peak at 222.1 that is corresponding to base ion peak with 99 % intensity. This compound also gives other peaks at 791.2, 730, 503.2, 350, 496.3 and 154.1. The spectrum exhibited other peaks assignable to various fragments arising from the thermal cleavage of the complexes. The peak intensities give an idea of a stability of the fragment that showed at 222.1.
Compound (B) exhibit molecular ion peak at 891.5 and other fragments of this compound is arised at 821.3, 510.3, 400.7, 345.1, 300.3, 296.3 and 106.0. This compound give base ion peak at 103.2 with 80 % intensity. In addition to the molecular ion peaks, the spectrum exhibited other peaks assignable to various fragments arising from the thermal cleavage of the complexes. The peak intensities given an idea of a stability of the fragment that showed at 103.2.
Compound (C) exhibited molecular ion peak at 846.3 and other peaks of the compound 6d observed at 796.1, 490.1, 400.2, 283.4, 223.1, 103.0 etc. This compound observed maximum intensities at 283.4 that are corresponding to base ion peaks. In addition to the molecular ion peak, the spectrum exhibited other peaks assignable to various fragments arising from the thermal cleavage of the complexes. The peak intensities given an idea of a stability of the fragment that showed at 283.4.
Compound (D) showed molecular ion peak at 831.1 that is corresponding to molecular ion peak and it gives base ion peak at 192.1 with 100 % intensity. The other peak of compound 6e observed at 665.1, 405.2, 361.4, 340.3, 275.3, 192.1, 104.0 etc. In addition to the molecular ion peak, the spectrum exhibited other peaks assignable to various fragments arising from the thermal cleavage of the complexes. The peak intensities given an idea of a stability of the fragment that showed at 192.1.
Compound (E) give molecular ion peak at 849.1 and other peaks of the compound at 732.1, 551.1, 378.1, 338.2, 219.1, 249.1 etc. The compound 6f observed base ion peaks at 378.1. In addition to the molecular ion peak, the spectrum exhibited other peaks assignable to various fragments arising from the thermal cleavage of the complexes. The peak intensities given an idea of a stability of the fragment that showed at 378.1.
Table 1: Physical and Analytical data of Copper (II) complexes of Schiff base molecular adducts
| S.No. | Compounds | Molecular Formula | Color | Yield | M.P. (°C) | Mol.wt. | Molar conductance Ω-1cm2mol-1 | Analysis (%) Found (Calcd.) | λ max | ||
| C% | H% | N% | |||||||||
| 1. | A | [Cu(C42H50N10O2)]Cl2 | Green | 65 | 225 | 860.54 | 46.5 | 57.84/58.56 | 5.97/5.81 | 15.76/16.26 | 365 nm |
| 2. | B | [Cu(C40H44N12O4)]Cl2 | Green | 76 | 240 | 890.54 | 38.2 | 53.56/53.89 | 4.73/4.94 | 17.51/18.86 | 390 nm |
| 3. | C | [Cu(C38H40N10Cl2)]Cl2 | Green | 40 | 310 | 841.54 | 53.3 | 54.21/54.18 | 4.26/4.75 | 16.79/16.63 | 386 nm |
| 4.. | D | [Cu(C40H46N10O2)]Cl2 | Cream | 60 | 165 | 832.54 | 23.1 | 57.12/57.64 | 5.89/5.52 | 16.82/16.81 | 395 nm |
| 5.. | E | [Cu(C38H40N12O4)]Cl2 | Greenish yellow | 48 | 230 | 862.54 | 26.2 | 51.78/52.86 | 4.77/4.63 | 19.37/19.47 | 410 nm |
Infrared Spectra
The main IR vibration bands of all copper complexes are listed in Table 2. Upon coordination, change in the frequency ν (C=N) and ν (N-H) wave numbers in comparison to the values found for the macrocyclic ethylene diammine ligands were observed for complexes a-f. They are consistent with the tetra dentate coordination of the ligand through the azomethine nitrogen atom.
Table 2: The Relevant IR peaks of copper (II) complexes of Schiff base molecular adducts
| S. No. |
Compounds |
ν(N-H) |
ν(Ar-H) |
ν (C=N) |
ν (Cu-N) |
| 1. |
A |
3299 |
3034 |
1581 |
530 |
| 2.. |
B |
3231 |
3034 |
1581 |
534 |
| 3. |
C |
3299 |
3038 |
1621 |
494 |
| 4. |
D |
3284 |
2965 |
1607 |
522 |
| 5. |
E |
3245 |
3030 |
1597 |
530 |
Compound (A) exhibit a peak on 3299 cm-1 which confirm that it contain ν (N-H) group and it show a band on 3034 cm-1 correspond to aromatic C-H ; compound exhibit a band on 1581 cm-1 which correspond to ν(C=N), the shifting of ν(C=N) band from 1650 to 1581; which confirmed the coordination occur through the azomethine group, the compound observed a band on 530 cm-1 indicates that a bond form in between ν(Cu-N). The occurrence of the ν (N-N) band at higher frequencies in the IR spectrum of the complexes as compared to those observed for the ligands, confirmed coordination through the azomethine nitrogen atom. The spectrum does not exhibit any band at the 1684 cm-1 frequency which was observed in compound 3(b) indicates that condensation occurs in between ν (C=O) group of ligand and NH2 group of ethylene diammine.
Compound (B) exhibit a peak on 3231 cm-1 which confirm that it contain ν (N-H) group and it show a band on 3034 cm-1 correspond to aromatic C-H ; compound exhibit a band on 1581 cm-1 which correspond to ν(C=N), the shifting of ν(C=N) band from 1640 to 1581; which confirmed the coordination occur through the azomethine group, the compound observed a band on 534 cm-1 indicates that a bond form in between ν(Cu-N). The occurrence of the ν (N-N) band at higher frequencies in the IR spectrum of the complexes as compared to those observed for the ligands, confirmed coordination through the azomethine nitrogen atom. The spectrum does not exhibit any band at the 1692 cm-1 frequency which was observed in compound 3(c) indicates that condensation occurs in between ν (C=O) group of ligand and NH2 group of ethylene diammine.
Compound (C) exhibit a peak on 3299 cm-1 which confirm that it contain ν (N-H) group and it show a band on 3038 cm-1 correspond to aromatic C-H ; compound exhibit a band on 1621 cm-1 which correspond to ν(C=N), the shifting of ν(C=N) band from 1650 to 1621; which confirmed the coordination occur through the azomethine group, the compound observed a band on 494 cm-1 indicates that a bond form in between ν(Cu-N). The occurrence of the ν (N-N) band at higher frequencies in the IR spectrum of the complexes as compared to those observed for the ligands, confirmed coordination through the azomethine nitrogen atom. The spectrum does not exhibit any band at the 1684 cm-1 frequency which was observed in compound 4(a) indicates that condensation occurs in between ν (C=O) group of ligand and NH2 group of ethylene diammine.
Compound (D) exhibit a peak on 3284 cm-1 which confirm that it contain ν (N-H) group and it show a band on 2965 cm-1 correspond to aromatic C-H ; compound exhibit a band on 1607 cm-1 which correspond to ν(C=N), the shifting of ν(C=N) band from 1651 to 1607 cm-1; which confirmed the coordination occur through the azomethine group, the compound observed a band on 522 cm-1 indicates that a bond form in between ν(Cu-N). The occurrence of the ν (N-N) band at higher frequencies in the IR spectrum of the complexes as compared to those observed for the ligands, confirmed coordination through the azomethine nitrogen atom. The spectrum does not exhibit any band at the 1690 cm-1 frequency which was observed in compound 4(b) indicates that condensation occurs in between ν (C=O) group of ligand and NH2 group of ethylene diammine.
Compound (E) exhibit a peak on 3245 cm-1 which confirm that it contain ν (N-H) group and it show a band on 3030 cm-1 correspond to aromatic C-H ; compound exhibit a band on 1597 cm-1 which correspond to ν(C=N), the shifting of ν(C=N) band from 1655 to 1597 cm-1 ; which confirmed the coordination occur through the azomethine group, the compound observed a band on 530 cm-1 indicates that a bond form in between ν(Cu-N). The occurrence of the ν (N-N) band at higher frequencies in the IR spectrum of the complexes as compared to those observed for the ligands, confirmed coordination through the azomethine nitrogen atom.
1H-NMR Spectra
The 1H-NMR spectra of the complexes were obtained in CDCl3 at room temperature using TMS as an internal standard. The main peaks observed in spectra are given in table 3.
Table 3: The Relevant 1H-NMR peaks of copper (II) complexes of Schiff base molecular adducts
| S.No. | Compounds |
δ CH3 |
δ CH2 |
δ NH |
δ C6 H5 |
| 1. |
A |
2.56 |
2.67-2.68 |
3.96-4.02 |
6.7-6.96 |
| 2. |
B |
1.1 |
2.1-2.25 |
3.60-3.67 |
7.35-8.03 |
| 3. |
C |
2.1 |
2.23-2.34 |
3.11-3.12 |
6.81-7.9 |
| 4. |
D |
1.8 |
2.48-2.50 |
3.42 |
7.01 – 7.36 |
| 5 |
E |
2.15 |
2.51 |
3.18-3.5 |
7.45-8.11 |
Complex (A) observed a peak at δ 2.56 ppm in 1H-NMR spectrum, which is corresponding to methyl group; aliphatic –CH2 group obtained singlet in between δ 2.67 – 2.68 ppm. The 1H-NMR spectrum of metal complexes showed signals corresponding to –NH at the δ 3.96 – 4.02 ppm. The multiplets observed in the region δ 6.7 – 6.96 ppm were assigned to the aromatic ring protons of carbohydrazone and the ethylene diammine moiety. The 1H-NMR spectrum of copper chelates confirmed the participation of imino –NH group in the coordination with copper ions. Some hydrogen atom values of δ were not observed precisely due to overlapping with the signals of the aromatic hydrogen atoms of carbohydrazone moiety. 1H-NMR integration and signal multiplicity were found to support the proposed structures.
Complex (B) a peak at δ 1.1 ppm in 1H-NMR spectrum, which is corresponding to methyl group; aliphatic –CH2 group obtained singlet at δ 2.1 – 2.25 ppm. The multiplets observed in the region δ 7.35 – 8.03 ppm were assigned to the aromatic ring protons of carbohydrazone and the ethylene diammine moieties. The 1H-NMR spectra of copper complexes showed signals corresponding to –NH at the δ 3.60 – 3.67 ppm. The 1H-NMR spectrum of copper chelates confirmed the participation of imino –NH group in the coordination with copper ions. Some hydrogen atom values of δ were not observed precisely due to overlapping with the signals of the aromatic hydrogen atoms of carbohydrazone moiety. 1H-NMR integration and signal multiplicity were found to proposed structure.
The 1H-NMR spectrum of complex (C) which showed singlet at δ 2.23 – 2.34 ppm that are correspond to aliphatic –CH2 group. Spectrum observed a peak at δ 2.1 ppm in 1H-NMR spectrum, which is corresponding to methyl group. The multiplets observed in the region δ 6.81 – 7.9 ppm were assigned to the aromatic ring protons of carbohydrazone and the ethylene diammine moieties. The 1H-NMR spectrum of copper complexes showed signals corresponding to –NH at the δ 3.11 – 3.12 ppm. The 1H-NMR spectrum of copper chelates confirmed the participation of imino –NH group in the coordination with copper ions. Some hydrogen atom values of δ were not observed precisely due to overlapping with the signals of the aromatic hydrogen atoms of carbohydrazone moiety. 1H-NMR integration and signal multiplicity were found to be in agreement with the proposed structure.
Complex (D) observed a peak at δ 1.8 ppm, which is corresponding to methyl group; aliphatic –CH2 group obtained singlet at δ 2.48 – 2.50 ppm in the spectrum. The multiplets observed in the region δ 7.01 – 7.31 ppm were assigned to the aromatic ring protons of carbohydrazone and the ethylene diammine moieties. The 1H-NMR spectrum of copper complexes showed signals corresponding to –NH at the δ 3.42 ppm. The 1H-NMR spectrum of copper chelates confirmed the participation of imino –NH group in the coordination with copper ions. Some hydrogen atom values of δ were not observed precisely due to overlapping with the signals of the aromatic hydrogen atoms of carbohydrazone moiety. 1H-NMR integration and signal multiplicity were found to be in agreement with the proposed structure.
Complex (E) observed a peak at δ 2.15 ppm in 1H-NMR spectrum, which are corresponding to methyl group; aliphatic –CH2 group obtained singlet at δ 2.51 ppm in the 1H-NMR spectrum. The multiplets observed in the region δ 7.45 – 8.11 ppm were assigned to the aromatic ring protons of carbohydrazone and the ethylene diammine moieties. The 1H-NMR spectrum of copper complexes showed signals corresponding to –NH at the δ 3.18 – 3.5 ppm. The 1H-NMR spectrum of copper chelates confirmed the participation of imino –NH group in the coordination with copper ions. Some hydrogen atom values of δ were not observed precisely due to overlapping with the signals of the aromatic hydrogen atoms of carbohydrazone moiety. 1H-NMR integration and signal multiplicity were found to be in agreement with the proposed structure.
In-vitro Antineoplastic Activity
MCF-7 (Human Breast carcinoma) cell line was procured from National Centre for Cell Sciences (NCCS), Pune, India. Stock cells were cultured in DMEM supplemented with 10% inactivated Fetal Bovine Serum (FBS), penicillin (100 IU/ml), streptomycin (100 mg/ml) and amphotericin B (5 mg/ml) in an humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with TPVG solution (0.2% trypsin, 0.02% EDTA, 0.05% glucose in PBS). The stock cultures were grown in 25 cm2 culture flasks and all experiments were carried out in 96 microtitre plates (Tarsons India Pvt. Ltd., Kolkata, India).
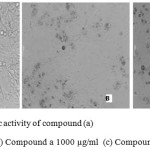 |
Figure 2: Anti neoplastic activity of compound (a) Control – MCF 7 (b) Compound a 1000 µg/ml (c) Compound a 500 µg/ml Click here to View figure |
Preparation of Test Solutions
For Cytotoxicity studies, each weighed test drugs were separately dissolved in distilled DMSO and volume was made up with DMEM supplemented with 2% inactivated FBS to obtain a stock solution of 1 mg/ml concentration and sterilized by filtration. Serial two fold dilutions were prepared from this for carrying out cytotoxic studies.
Determination of cell viability by MTT Assay
The ability of the cells to survive a toxic medium has been the basis of most Cytotoxicity assays. This assay is based on the assumption that dead cells or their products do not reduce tetrazolium. The assay depends both on the number of cells present and on the mitochondrial activity per cell. The principle involved is the cleavage of tetrazolium salt 3-(4, 5 dimethyl thiazole-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) into a blue coloured product (formazan) by mitochondrial enzyme succinate dehydrogenase. The number of cells was found to be proportional to the extent of formazan production by the cells used.
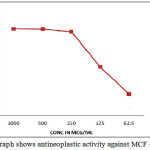 |
Figure 3: Graph shows antineoplastic activity against MCF – 7 cell line Click here to View figure |
The all compounds were evaluated for their effectiveness against the human breast cancer cell line MCF-7 using MTT cytotoxicity assay. For comparison purpose, the cytotoxicity of the standard anti-breast cancer drug Tamoxifen was used under the same experimental conditions. The IC50 value was calculated using MTT assay, as shown in Table 4 . The results revealed that the activity of compounds increases by the presence of bulky groups bonded to N4 of the ethylene diammine moiety. The compounds were found to have high activity. The similarity in the values of IC50 for the Cu (II) complexes is evidence in favor of the same biochemical action mechanism. In fact, the literature reports that Cu (II) complexes of ethylene diammine derivatives are able to bind DNA in vitro and present enhanced capacity to form inter-strand cross links as compared to cisplatin. Result revealed that compounds were exhibit potent activity against the MCF – cell line.
Table 4: Cytotoxic activity of Cu (II) complexes of Schiff base molecular adducts against MCF-7 breast cancer cell line
| S.No. | Compounds | Test Conc. (%) | % Cytotoxicity | CTC50 (µg/ml) |
| 1. | A | 1000500250
125 62.5 |
78.62±0.278.43±1.076.04±0.644.08 ±4.2
19.52±5.9 |
145.22±8.9 |
| 2. | B | 1000500250125
62.5 |
78.68±0.378.50±1.076.06±0.644.09±4.4
19.58 ± 6.0 |
146.24±8.9 |
| 3. | C | 1000500250125
62.5 |
78.65±0.478.52±1.576.06±0.844.09±4.1
19.52±5.3 |
144.00±8.7 |
| 4. | D | 1000500250125
62.5 |
78.76±0.3478.58±1.476.24±0.544.05±4.6
19.56±5.9 |
145.00±8.7 |
| 5. | F | 1000500250125
62.5 |
78.64±0.678.50±1.576.09±0.744.09±4.6
19.53±5.4 |
145.20±8.8 |
In-vitro Antibacterial Activity
The all copper (II) complexes were also evaluated for their potential antibacterial activity against B. subtilis (MTCC 1134), S. aureus (MTCC 3160), E. coli (MTCC 50) and P. aeruginosa (MTCC 1034). Tables 5 highlight the antibacterial activity of complexes a-e against B. subtilis, S. aureus, E. coli and P. aeruginosa as observed by disc-diffusion method. The high antibacterial activity of copper (II) complexes may be due to coordination and chelation which tend to make copper complexes act as powerful and potent bacteriostatic agents, thus inhibiting the growth of the bacteria. In a complex, the positive charge on the copper is partially shared with the donor atoms present in the ligands and there may be delocalization of π electrons over the whole chelate. The increased activity of the metal chelates can be explained on the basis of chelation theory. The result of the this series revealed that the all copper (II) complexes contain significant antibacterial activity against two gram positive bacteria and two gram negative bacteria. All the copper (II) complexes exhibit higher antibacterial activity against the P. aurogenosa bacteria.
Table 5: Invitro antibacterial activities of all compounds against gram positive and gram negative bacteria
| Compounds | Zone of inhibition (in mm) and Concentration 1mg/ mL of various strains | |||
|
E. coli |
P. aerogenosa |
B. subtilis |
S. aureus |
|
|
A |
13 |
22 |
15 |
12 |
|
B |
14 |
25 |
15 |
13 |
|
C |
9 |
18 |
6 |
8 |
|
D |
10 |
16 |
5 |
6 |
|
E |
8 |
10 |
10 |
9 |
|
Tetracyclin(Standard) |
10 |
6 |
20 |
22 |
Table 4 revealed that all the copper (II) complexes exhibit significant activity against the tested two gram positive and two gram negative bacteria. The orders of antibacterial activity of the complexes against the E. coli are as follows Compound c > Compound b > Compound a > Compound e > Compound d
The orders of antibacterial activity of the complexes against the P. Aerogenosa are as follows Compound c> Compound a> Compound b> Compound d > Compound e. The orders of antibacterial activity of the complexes against the B. subtilis are as follows Compound b= c> Compound a = Compound d> . The orders of antibacterial activity of the complexes against the S. aureus are as follows Compound c> Compound b> Compound a> > d> e . All the copper (II)
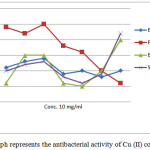 |
Figure 4: Graph represents the antibacterial activity of Cu (II) complexes Click here to View figure |
In-vitro Antioxidant Activity of Copper (II) Complexes of Schiff base molecular adducts
The copper (II) complexes have been suggested as promising agents for the diagnosis and treatment of different disease. All compounds showed significant free radical scavenging action against peroxide induced release of free radicals at varying concentrations (200-1000 µg/ml). Ascorbic acid was used as a reference standard. The % scavenging is shown in Table. In addition, some complexes have been suggested as a potential SOD mimics, mainly because of their high thermodynamic stability. 6 revealed that the all compounds showed the significant antioxidant activity at the concentration of 1000 µg / ml.
Table 6: In-vitro free radical scavenging effect of copper (II) complexes of Schiff base molecular adducts
| S.No. | Compounds |
% Scavenging of triplicates |
||||
|
200 µg/ml |
400 µg/ml |
600 µg/ml |
800 µg/ml |
1000 µg/ml |
||
| 1. |
A |
25 |
39.5 |
45.3 |
46 |
74 |
| 2. |
B |
22.5 |
37.9 |
52.2 |
44.3 |
73.3 |
| 3. |
C |
14.6 |
34.13 |
57.3 |
42.5 |
72.0 |
| 4 |
D |
21.8 |
35.54 |
62.1 |
42.5 |
72.63 |
| 5. |
E |
20.17 |
35.67 |
60.1 |
42.65 |
72.63 |
| 6. | Ascorbic acid |
50 |
65.4 |
68.2 |
75.3 |
80.6 |
The antioxidant activity of all the copper (II) complexes given in table 6, the order of antioxidant activities of all complexes are as: Compound b > Compound c > Compound f = Compound e> Compound d > Compound a. All the copper (II) complexes showed the significant antioxidant activity against. All the copper (II) complexes exhibit significant antioxidant activity but less than control ascorbic acid, compound e exhibit highest antioxidant activity due to the presence of nitro group in Schiff base molecular adduct.
Conclusion
This paper describes the Synthesis, Spectroscopic Characterization and Pharmacological Evaluation of Schiff Base molecular Adducts of Copper metal. . All Cu (II) compounds were synthesized and well characterized in detail by FTIR, 1HNMR, 13CNMR and LC-MS analysis. The Cu (II)compounds were in a distorted octahedral environment with the ligand having a tetradentate nitrogen chelating motif. All Cu (II) compounds showed significant in-vitro cytotoxic activity against human breast cancer cell lineMCF-7. Further studies would entail studying the in vitro cytotoxic effect of the complexes against other cancer celllines viz. lung, colon, ovarian etc., followed by in vivo studies in animal models as well as the in vitro effect of thecomplexes on various normal cell lines. More detailed studies are needed to understand the mechanisms of action at the cellular level and the role of the metal.Cell shrinkage and rounding, membrane blebbing, chromatin condensation and nuclear fragmentation are importantcharacteristics of apoptosis. In our study, prominent morphological changes, which are associated with apoptosisviz. live cell rounding, cell shrinkage and nuclear fragmentation, were observed when MCF-7 breast cancer cell line when treated with the compounds for 10 h. The data reported in this article might prove helpful guide for medicinal chemists working in this area. Investigation of antibacterial screening data revealed that compounds exhibited significant antibacterial activityagainst B. subtilis, P. aerogenosa , S. aureus and E. coli. All compounds were found to possess potent antioxidant activity in the range of 80-90% when screened for their radical scavenging activity against DPPH compound . Many present day diseases are reported to be due to an impaired balance of the pro-oxidant-antioxidant homeostatic phenomenon in the body. Pro-oxidant conditions dominate either on account of increased generation of free radicals caused by excessive oxidative stress, or due to poor scavenging in the body caused by depletion of the dietary antioxidants. Reactive oxygen species differ significantly in their interactions and can cause extensive cellular damage such as nucleic acid strand scission, modification of polypeptides, lipid peroxidation etc . Antioxidants are the first line of defense against free radical damage, and are critical for maintaining optimum health. The need for antioxidants becomes even more critical with increased exposure to free radicals. As part of a healthy lifestyle and a well-balanced, wholesome diet, antioxidant supplementation is now being recognized as an important means of improving free radical protection.The macrocyclic ligands are highly significant in bioinorganic chemistry, catalysis as well as extraction of metal ions etc. Schiff base molecular adducts with transition metal ions show some interesting properties and biological functions such as being models for metalloproteinase and oxygen carrier systems. Keeping the above facts in mind and in continuation of our research work, the present paper reports the Synthesis, Spectroscopic Characterization and Pharmacological Evaluation of Schiff Base molecular Adducts of Copper metal These complexes have the potential to emerge as leading candidates for drug development, if studied and screened further for their in vivo effects.
Acknowledgements
The authors are thankful to the Chancellor, Vice Chancellor, Managing Director, ITM University, Gwalior, for their support and co-operation. RK is grateful to MPCST for providing financial assistance in the form of grant no. (Council order No. 4566/ Cst/ R&D/2010).
References
- Z. Cimerman, S. Miljanić, and N. Galić, Croatica Chemica Acta, 2000, 73, 81–95,.
- H. Schiff, Justus Liebigs Annalen Der Chemie. 1864, 131, 118–119.
CrossRef - D. N. Dhar and C. L. Taploo, Journal of Scientific and Industrial Research, 1982, 41, 8, 501–506.
- B. S. Sathe, E. Jaychandran, V. A. Jagtap, and G. M. Sreenivasa, International Journal of Pharmaceutical Research and Development, 2011, 3, 164–169.
- S. M. Sondhi, N. Singh, A. Kumar, O. Lozach, and L. Meijer, Bioorganic and Medicinal Chemistry, 2006, 14, 3758–3765.
CrossRef - Pandey, D. Dewangan, S. Verma, A. Mishra, and R. D. Dubey, ”International Journal of ChemTech Research, 2011, 3, 178–184.
- Chandramouli, M. R. Shivanand, T. B. Nayanbhai, B. Bheemachari, and R. H. Udupi, Journal of Chemical and Pharmaceutical Research, 2012, 4, 1151–1159.
- R. P. Chinnasamy, R. Sundararajan, and S. Govindaraj, Journal of Advanced Pharmaceutical Technology and Research, 2010, 1, 342–347.
CrossRef - K. Mounika, B. Anupama, J. Pragathi, and C. Gyanakumari, Journal of Scientific Research, 2010, 2, 513–524.
- P. Venkatesh, Asian Journal of Pharmaceutical and Health Sciences, 2011, 1, 8–11.
- K. Chaubey and S. N. Pandeya,” International Journal of PharmTech Research, 2012, 4, 590–598.
- T. Aboul-Fadl, F. A. Mohammed, and E. A. Hassan,” Archives of Pharmacal Research, 2003, 26,778–784.
- R. Miri, N. Razzaghi-asl, and M. K. Mohammadi, Journal of Molecular Modeling, vol. 2013, 19,727–735.
- S. M. M. Ali, M. Abul Kalam Azad, M. Jesmin et al., Asian Pacific Journal of Tropical Biomedicine, 2012, 2, 438–442.
CrossRef - Wei, N. Li, G. Lu, and K. Yao, Science in China B, 2006, 49, 225–229.
CrossRef - P. G. Avaji, C. H. Vinod Kumar, S. A. Patil, K. N. Shivananda, and C. Nagaraju, “ European Journal of Medicinal Chemistry, 2009, 44, 3552–3559.
CrossRef - K. N. Venugopala and B. S. Jayashree, Indian Journal of Heterocyclic Chemistry, 2003,12, 4, 307–310.
- K. Vashi and H. B. Naik, European Journal of Chemistry, 2004, 1, 272–276.
- S. Li, S. Chen, S. Lei, H. Ma, R. Yu, and D. Liu, Corrosion Science, 1999, 41, 1273–1287,
CrossRef - Z. H. Chohan, M. Praveen, and A. Ghaffar, Metal-Based Drugs, 1997, 4, 267–272.
CrossRef - S. Ershad, L. Sagathforoush, G. Karim-Nezhad, and S. Kangari, International Journal of Electrochemical Science, 2009, 4, 846–854,
- F. Tisato, F. Refosco, and G. Bandoli, Coordination Chemistry Reviews, 1994, 135-136, 325–397.
CrossRef - Jarrahpour, D. Khalili, E. De Clercq, C. Salmi, and J. M. Brunel, Molecules, 2007, 12, 1720–1730,
CrossRef - Bhattacharya, V. C. Purohit, and F. Rinaldi, Organic Process Research and Development, 2003, 7, 254–258.
CrossRef - G. Bringmann, M. Dreyer, J.H. Faber, P.W. Dalsgaard, D. Staerk, J.W. Jaroszewski, et al. J Nat Prod, 2004, 67,743–74.
CrossRef - A.O. de Souza, F.C.S. Galetti, C.L. Silva, B. Bicalho, M.M. Parma, S.F. Fonseca, et al. Quim Nova, 2007, 30, 1563–1566.
- Z. Guo, R. Xing, S. Liu, Z. Zhong, X. Ji, L. Wang, et al. Carbohydr Res, 2007, 342, 1329–1332.
CrossRef - Y. Zheng, K. Ma, H. Li, J. Li, J. He, X. Sun, et al . Catal Lett, 2009, 128 (3-4), 465–474.
CrossRef - R.B. Moffett, N. Rabjohn (Ed.), Organic syntheses, John Wiley & Sons, Inc., New York (USA) 1963, 4, 605–608.
- K. Taguchi, F.H. J Org Chem, 1971, 36, 1570–1572.

This work is licensed under a Creative Commons Attribution 4.0 International License.









