Synthesis, Spectral Characterization and Anticancer Studies of Some Metal(II) Complexes Derived from Imidazole-2-Carboxaldehyde with 2-Amino-3-Carboxyethyl-4,5-Dimethylthiophene
Sonia Mol Joseph1, C. Justin Dhanaraj2, J. Joseph3 and R. Selwin Joseyphus1
1Department of Chemistry, Mar Ivanios College (Autonomous), Nalanchira, Thiruvananthapuram-695015, Kerala, India.
2Department of Chemistry, University College of Engineering, Anna University Constituent College, Nagercoil-629004, Tamil Nadu, India.
3Department of Chemistry, Noorul Islam University, Kumaracoil-629180, Tamil Nadu, India.
Corresponding Author E-mail: soniamol.joseph@mic.ac.in
DOI : http://dx.doi.org/10.13005/ojc/330351
Imidazole-2-carboxaldehyde was condensed with 2-amino-3-carboxyethyl-4,5-dimethyl thiophene in 1:1 molar ratio yielded Schiff base ligand. Cobalt(II), nickel(II), copper(II) and zinc(II) complexes of the ligand were synthesized and characterized structurally using elemental analysis, molar conductance, magnetic moment, infrared and electronic spectra. The ligand structure was confirmed by proton nuclear magnetic resonance spectrum. The geometry exhibited by the complexes was proposed using magnetic and electronic spectral data. Thermal analysis was carried out to ascertain the thermal stability of the compounds. The fluorescence spectral analysis were investigated at different solvents for the ligand and copper(II) complex. Using powder X-ray diffraction measurements, the grain size was determined. The scanning electron microscope images indicate the surface morphology of the complexes. The antibacterial and antifungal activities were screened by disk diffusion method designed by Kirby-Bauer. In vitro anticancer studies were carried out, by MTT assay for human cervical carcinoma cell line.
KEYWORDS:Schiff base; magnetic moment; infrared spectra; electronic; surface morphology; anticancer
Download this article as:| Copy the following to cite this article: Joseph S. M, Dhanaraj C. J, Joseph J, Joseyphus R. S. Synthesis, Spectral Characterization and Anticancer Studies of Some Metal(II) Complexes Derived from Imidazole-2-Carboxaldehyde With 2-Amino-3-Carboxyethyl-4,5-Dimethylthiophene. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Joseph S. M, Dhanaraj C. J, Joseph J, Joseyphus R. S. Synthesis, Spectral Characterization and Anticancer Studies of Some Metal(II) Complexes Derived from Imidazole-2-Carboxaldehyde With 2-Amino-3-Carboxyethyl-4,5-Dimethylthiophene. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33963 |
Introduction
Imidazole derivatives have wide biological and pharmaceutical applications such as antidiabetic, antihypertensive and anti-inflammatory activities1. Moreover, the substituted imidazoles are synthetically important for the use of synthetic intermediates such as, catalysts and asymmetric catalysis2. Thiophene derivatives are one of the most extensive dynamic research areas and exist as natural and synthetic pharmaceuticals3. Derivatives of thiophene play an active drug; therefore it is used in therapy and biodiagonostics4. Schiff base ligands prepared by the reaction between aldehydes and imines are considered to be ‘‘privileged ligands’’, because they can bind with diverse metals, stabilize them in various states. Schiff base and additional electron donor (nitrogen and oxygen) atoms are usually used as the ionophore in fluorescent sensor for determining several cations5-7. The novel nitrogen-containing heterocyclic compounds and their complexes may act as suitable catalysts in many chemical reactions8. Heterocyclic compounds containing amino or nitrogen atom of azomethine group and phenolic oxygen atoms that have rendered more interest to the research workers9-14. Schiff base complexes of various 3d metal ions have been investigated for their coordinating capability. Pharmacological6 and toxicological activities are shown by modified drugs when applied in the form of metal complex systems12. The fluorescent sensors for metal ions have become globally used in recent years to the ease in handling and high sensitivity. In continuation of our research work15,16, this article deals about the synthesis, structural elucidation and biological studies of Schiff base metal complexes. These synthesized metal complexes may be applied to pharmaceuticals and in drug designing applications.
Materials and Methods
Analar grade solvents and chemicals were used for this study. Metal salts and ligands used were obtained from Merck. Microanalysis (C, H, N) was done using Carlo Erba EA1108 elemental analyzer. Molar conductance was measured with digital conductivity meter. The magnetic susceptibility measurements were made using Faraday balance method. Diamagnetic corrections were done by Pascal`s constants and Hg[Co(SCN)4] was used as calibrant. Infrared spectra of ligand and its complexes were recorded on a Shimadzu IR Affinity-1S FT-IR Spectrophotometer. UV-Visible spectra of the metal complexes were recorded on a Systronics double beam UV-VIS spectrophotometer. The 1H-NMR spectrum of the ligand was recorded with BRUKER Avance –III 300 MHz NMR spectrometer, using internal standard tetramethylsilane and DMSO-d6 as solvent. The thermogravimetric analysis was studied using Shimadzu TGA-50H thermal analyzer in nitrogen atmosphere with 10°C min-1 heating rate. Fluorescence spectra of ligand and copper(II) complex was investigated by PTI QM-2000-4 Bench-Top model steady state spectrophotometer. Powder X-ray diffraction measurements were conducted using Rigaku D/Max-B X-ray diffractometer. Scanning electron microscope was recorded in Hitachi S5500 SEM instrument analyzer.
Synthesis of Schiff Base Ligand
The ligand precursor 2-amino-3-carboxyethyl-4,5-dimethylthiophene was synthesized by Gewald synthesis17. The starting material 2-Amino-3-carboxyethyl-4,5-dimethylthio phene (1 mmol) was dissolved with methanol. To this a solution of imidazole-2-carboxaldehyde (1 mmol) dissolved in methanol was added dropwise. The mixture was stirred magnetically and refluxed for 3 h. The resulting solutions volume was reduced in a boiling water bath, cooled at room temperature. The resulting compound was washed with ether, recrystallized using ethanol. Good yield of about 76% was obtained. The purity of the ligand was checked by thin layer chromatographic technique.
Synthesis of Metal Complexes
The ligand (1 mmol) was dissolved in methanol. To this, cobalt/ nickel/ copper/ zinc(II) chlorides (1 mmol) dissolved in methanol was added dropwise under magnetic stirring. The above mixture was refluxed for 2 h. The solid products was washed with ether, filtered, and dried in vacuum desiccator. Yield of about 66-72% was obtained for the metal complexes. Purity of the complexes was checked using thin layer chromatographic technique.
Results and Discussion
The synthesized ligand and metal(II) complexes are found to be stable in air. Elemental analysis and physical parameters agree well with the calculated values in accordance with their formulae. Metal complexes was formulated as [MLX2], where M= CoII/NiII/CuII/ ZnII, L = Schiff base ligand, and X= Cl. The low molar conductance value determined in dimethylformamide falls in the range 10-19 ohm-1 cm2 mol-1 suggesting the non-electrolytic nature18 of metal complexes. For the ligand the ultraviolet absorption band was observed at 354 nm, corresponds to n-π* transitions of aromatic ring and azomethine chromophore. The 1H- NMR ligand spectrum exhibits the azomethine proton (-HC=N-) signal appeared at δ 8.4 ppm. The multiplet signals were also observed for aromatic protons (-CH=CH-) at δ 7.4-8.0 ppm and two methylene (-CH2-) protons at δ 3.8-4.2 ppm. The assigned structure of ligand is displayed in Scheme 1.
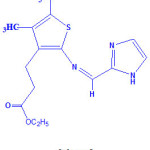 |
Scheme 1
|
Infrared Spectra
The stretching frequency band of azomethine group (-HC=N-) occurred at 1618 cm-1 in the ligand. This band was shifted during complex formation to lower frequency region 1612 – 1609 cm-1 in the spectra of the complexes indicating coordination of azomethine nitrogen atom to the metal ion. The ester carbonyl ν(C=O) stretching frequency band appeared at 1640 cm-1 in the ligand was shifted down about 25 cm-1 in the metal complexes indicates the binding of ester carbonyl oxygen atom with the metal ion19. Also, the new stretching frequency bands were seen at 532-512 cm-1 ν(M-O), 410-405 cm-1 ν(M-N), and 295-245 cm-1 ν(M-Cl) vibrations respectively. The infrared spectral results demonstrate that the ligand act as neutral bidentate manner.
Electronic Spectra and Magnetic Measurements
Cobalt(II) complex displays an absorption band at 605 nm, corresponds to tetrahedral environment19 of ligand around metal ion, this corresponds to the transition 4A2(F) → 4T1(P). The cobalt(II) complex exhibits spin only magnetic moment value of 4.66 BM, corresponds to tetrahedral geometry. The nickel(II) complex displays only one absorption peak at 482 nm, this is due to d-d transition of nickel(II) caused by square planar arrangement of ligand field. Hence square planar geometry was assigned to this complex. The electronic spectrum of copper(II) complex (Fig. 1) shows a broad absorption band at 661 nm, this band corresponds to the d-d transition of copper(II) complex in square planar geometry. Zinc(II) complex is diamagnetic, therefore this complex would have tetrahedral geometry as predicted.
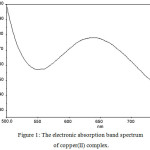 |
Figure 1: The electronic absorption band spectrum of copper(II) complex. |
Thermogravimetric Analysis
The thermal decomposition and thermal stability of metal complexes were estimated by thermal studies. The thermogravimetric curve displays three stages of decomposition. First stage decomposition noticed may be due to loss of thiophene moiety. Second stage decomposition was seen above 200oC may be due to the loss of imidazole moiety. Above 500oC only the metal residue remain20. The obtained results correlated well with theoretical formula calculation predicted from analytical data.
Fluorescence Spectra
The photoluminescence studies of the Schiff base (Fig. 2a) and copper(II) complex (Fig. 2b) were investigated at room temperature for 10-4 M solution in various solvents. Excitation and emission slit widths were fixed at 10 nm with a scan speed of 500 nm/min. The excitation spectrum of Schiff base shows a maximum excitation at 490 nm with an emission band at 450 nm. Schiff base systems normally exhibit fluorescence spectrum due to intra-ligand transitions21. At the concentration of 10-4 M solution in methanol, acetonitrile and dimethylsulphoxide solvents shows an excitation bands observed at 475-500 nm in the fluorescence spectra. The quenching spectrum was seen for copper(II) complex at 450 nm. The results show that the ligand has potential activity than the complex. The strongest quenching has been obtained for copper(II) complex.
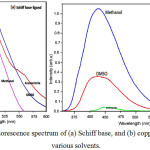 |
Figure 2a,b: Fluorescence spectrum of (a) Schiff base, and (b) copper(II) complex at various solvents. |
X-ray Diffraction
The powder X-ray diffraction were recorded at 2θ = 10-80° range. Sharp crystalline peaks were observed in the metal complexes, demonstrating the nanocrystalline behaviour22. From observed dXRD patterns, the average grain sizes of the cobalt(II), nickel(II), copper(II), and zinc(II) complexes were determined using Scherer’s formula. The grain size of the metal complexes was found to be 67, 77, 41 and 60 nm respectively suggest that metal complexes are in nanocrystalline phase.
Scanning Electron Microscope
For the metal complexes, the surface morphology was investigated by scanning electron microscope is shown in Fig. 3. The micrograph images showed irregularly shaped particles with micro crystalline structure for the metal complexes. Zinc(II) complex has cauliflower like morphological structure. Agglomerated size with controlled morphological images was observed for cobalt(II), nickel(II), copper(II) and zinc (II) complexes. Powder X-ray diffraction patterns of the Schiff base complexes has grain size less than 77 nm with controlled morphology.
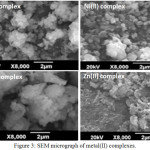 |
Figure 3: SEM micrograph of metal(II) complexes. |
Antimicrobial Activity
The bacterial and fungal species were tested by Kirby-Bauer disk diffusion method23. A. niger, C. albicans, S. aureus, P. aeruginosa, E. coli and K. pneumoniae were used as fungal and bacterial strains. Nystatin and chloramphenicol were used as standards. The test solutions were prepared in dimethylformamide solvent, also used as control. The ligand and complex results were compared with the standards and the inhibition zone diagram is shown in Fig. 4. In the present investigation, the results indicate that the complexes depict more growth inhibition potential than the ligand. It has been observed that the metal complexes are biologically active and chelation enhances their activity. A possible mode of toxicity will be based on chelation theory24. However, the zone of inhibition (mm) of ligand and its complexes varies with organisms as well as metal ions. Metal complexes moderately inhibited the growth of gram negative and positive bacterial strains and fungal activities.
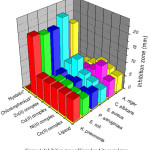 |
Figure 4: Inhibition zone of ligand and its complexes. |
Anticancer Activity
Anticancer activity of the synthesized complexes was screened on the human cervical carcinoma (HeLa) cells by MTT assay. The results in terms of IC50 values were compared with the literature25-27 reports. The concentration range used was 0.1-100 µM for the complexes. MTT assay results clearly indicate that the copper(II) and zinc(II) complexes show very good inhibitory effect on the growth of HeLa cells. The lowest IC50 value of 7.35 µM was seen in copper(II) complex, zinc(II) complex also exhibits IC50 value of 8.33 µM and cobalt(II) complex has IC50 value of 8.35 µM. The ligand and its nickel(II) complex (8.39 µM) showed lowest growth inhibitory activity towards HeLa cells (>100 µM).
Conclusions
The synthesized metal complexes are non-electrolytes in nature. The infrared data indicate that the Schiff base behaves as bidentate manner and coordinating via nitrogen atom of azomethine group and ester carbonyl oxygen atom. Magnetic data and electronic spectral results reveal different coordination environments of metal ions (square planar geometry for nickel(II) and copper(II) complexes, whereas tetrahedral geometry for cobalt(II) and zinc(II) complexes). Thermal results suggest that the metal complexes are thermally more stable than the ligand. The fluorescence spectral analysis of optical emission indicate that copper(II) complex exhibits strong fluorescence behaviour as compared with the ligand. Powder X-ray diffraction patterns pointed out the nanocrystalline nature of the metal complexes. SEM micrographs show agglomerated morphology for the metal complexes. Antimicrobial studies highlight more activity was present for metal complexes than the ligand. Except nickel(II) complex, all other metal complexes possess potent anticancer activity, whereas copper(II) complex exhibits maximum activity.
Acknowledgements
Authors are thankful to Noorul Islam University, Kumaracoil for extending the research activities for this work.
References
- Jigar, A.M.; Chetan B.S.; Lin, L.; Hai-Liang, Z. Biorg. Med. Chem. Lett., 2014, 24(7), 1734-1736.
CrossRef - Naeema, H.Y.; Hoda, A.E.; Mohamed, G. Mat. Sci. Eng. C, 2017, 75(1), 1059- 1067.
- Hassan, K.; Masoumeh, M.; Amir, S.; Leila, H.; Fariba, M.; Robert, W.G. Polyhedron, 2017, 127(8), 345-354.
- Shinu, C.; Subir, S. Biomed. Pharm., 2017, 89, 162-176.
CrossRef - Milan, M.; Mirjana, M.; Desanka, B.; Sanja, M.; Neda, N.; Vladimir, M.; Nenad, V.; Slobodan, S.; Slavica, S. Int. J. Mol. Sci., 2011, 12, 2822-2841.
CrossRef - Suresh, T.; Arunima, V.; Atin, K.; Sandeep, G.; Prarthana, V.R.; Ganesh, R.K. Acta Pol. Pharm., 2010, 67, 423-427.
- Nareshkumar, J.; Jiayi, X.U.; Ramesh, M.K.; Fuyong, D.U.; Guo J.Z. J. Med.Chem., 2009, 52, 7544-7569.
CrossRef - Mori, J.; Iwashima, M.; Takeuchi, M.; Saito, H. Chem. Pharm. Bull., 2006, 54, 391-396.
CrossRef - Brahma, S.; Ray P.; Singha, R.; Ray , J .K, Asian J Chem., 2016, 28(5), 1035-1038
CrossRef - Al-Razaq, E.A.; Buttrus, N.; Mohammed, E.H.; Jbara, A.A, Orient. J. Chem., 2016, 32(1), 137-148.
CrossRef - Khairy, A.M.; Mohsen, M.A.; Yahia, A.M.; Basyouni, W.M.; Samir, Y.A. World J. Chem., 2009, 4, 161-170.
- Joseph J, Rani, G.A.B. Appl. Biochem. Biotechol., 2014, 172(2), 867-890.
CrossRef - Nair, M.S.; Arish, D. T. Indian I. Metals, 2011, 64, 287-292.
- Prabhu, M.; Parthiban, K.; Chakkravarthi, G.; Prabakaran, E.; Rajagopal G. Asian J. Chem., 2016, 28(8), 1661-1666.
CrossRef - Dhanaraj, C.J.; Johnson, J. J. Coord. Chem., 2015, 68, 2449 -2469.
CrossRef - Joseyphus, R.S.; Israr, U. H.; Naikoo, G. A. Asian J. Chem., 2016, 28(7), 1571- 1574.
CrossRef - Gewald, K.; Schinke, E.; Bottcher, H. Chem. Ber., 1966, 99, 94-100.
CrossRef - Geary W. J. Coord. Chem. Rev., 1971, 7, 81-122.
CrossRef - Nakamoto, K. “Infrared and Raman Spectra of Inorganic and Coordination Compounds Part B Applications in Coordination, Organometallic, and Bioinorganic Chemistry”, 6th Edn., John Wiley & Sons Ltd., Canada, 2009.
- Sanaa, M.E. J. Mol. Struct., 2017, 1134, 444-457.
CrossRef - Faith, A.; Ali, I.O.; Bayram, S. J. Mol. Struct., 2017, 1157(5), 387-395.
- D’Eye, R.W.M.; Waite, E. X-ray Powder Photography in Inorganic Chemistry, Butterworth’s Scientific Pub., UK, 1960.
- Tweedy, B.G. Phytopathology, 1964, 55, 910.
- El-Azab, I.H.; Khaled, M.K. Russ. J. Bioorg. Chem., 2015, 41, 421–436.
CrossRef - Li, T.; Zhang,J.; Pan, J.; Wu, Z.; Hu, D.; Song, B, Eur. J. Med. Chem., 2017, 125, 657-662.
CrossRef - Ren , S.; Wang, R.; Komatsu, K.; Bonaz-Krause, P.; Zyrianov, Y.; McKenna, C.; Csipke, E.C.; Tokes, Z.A.; Lien, E. J, J. Med. Chem., 2002, 45(2), 410–419.
CrossRef - Nikil, M.P.; Bhupendra, M.M.; Muthuraman, P.; Surendra, K.S.; Rahul, V.P. Chin. Chem. Lett., 2017, 28(3), 602-606.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









