Synthesis, Crystal Identification, and Thermal Studies of A New Organic-Inorganic Hybrid Yttrium(III) Complex With Squarate
Louiza Zenkhri and Ahmed Boutarfaia
Department of Chemistry, Faculty of Mathematics and Matter Sciences BP 511 Route Ghardaia, Ouargla, Algeria - 30 000.
Corresponding Author E-mail: louizazenkhri@yahoo.fr
DOI : http://dx.doi.org/10.13005/ojc/330345
During studies of possible synthesis routes to inking amines into crystal lattice of metals squarate complexes, the reaction of the squarate anions, amine and yttrium under acid conditions have led to an isotype of Eu(H2O)6(C4O4)(HC4O4).H2O. The synthesis and identification of the new complex with yttrium (III) is described. The crystal structure with Europium is already reported. This compound was identified by powder X-ray diffraction and its crystal structure properties are determined.
KEYWORDS:Squarate; hybrid material; RDX identification; diffractogram indexing
Download this article as:| Copy the following to cite this article: Zenkhri L, Boutarfaia A. Synthesis, Crystal Identification, and Thermal Studies of A New Organic-Inorganic Hybrid Yttrium(III) Complex With Squarate. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Zenkhri L, Boutarfaia A. Synthesis, Crystal Identification, and Thermal Studies of A New Organic-Inorganic Hybrid Yttrium(III) Complex With Squarate. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33752 |
Introduction
Metals-carboxylate family are one of the most important materials attracting researchers in the last decade [1][2][3][4][5] owing to their interesting molecular topologies[6]. They present a great interest follows their hybrid character and three-dimensional networks [7] [8] [9]. Recently, a large number of studies have been carried out on metal squarates with an open structure. The squarate dianion (C4O42-) can link metal ions in various coordination modes through the oxygen atoms [10].
In our own investigations, we are interested in the synthesis and characterization of complexes of yttrium square with templates such as ethylenediamine (Ed) [11], piperazine (pip), 1,4-diazabicyclo [ 2.2.2] octane (Dabco). The inking of the amines in the crystal lattices participates in the enlargement of the pores or the tunnels formed by the frameworks of these materials.
In the present study, we describe the synthesis, and spectroscopy, XDR identification and thermal behaviour of the new isotype.
Materials and Methods
All chemicals used were analytical reagents and purchased commercially. Diffraction powder experiments were performed at room temperature on a D8 Advence X-ray spectrometer. The sample for measurement was prepared by depositing the dried powder on a silicon sample holder. The structures were identified using DIFFRAC EVA V3.1 program [12]. The crystal lattice search was carried out with the Dicvol 06 program [13]. The program used for powder pattern simulation and molecular charts is a mercury program [14]. The crystallographic data file used number is 1194867. Thermogravimetry analyses were carried out on a STA 449 F3 Jupiter. Thermal decomposition was recorded for 32 mg samples in (N2) flow with a heating rate of 5°min-1 up to 550°C.
Synthesis of Y(H2O)6(C4O4)(HC4O4).H2O
A solution of yttrium nitrate hexahydrate (0.19 g) in water (5 ml) was added dropwise to a solution of (Ed) 0.4 ml or (Pip) 0.48 g or (Dabco) 0.22 g in distilled water (10 ml). The solutions immediately became turbid and were homogenized by the addition of a drop of HNO3 with stirring for 5 minutes at 40 ° C. Then, 0.1 g of squaric acid in (10 ml) water was added dropwise. The clear solutions formed were stirred for 4 h at room temperature, and then colorless crystals formed were filtered and washed with water and dried in air.
Results and Discussion
X-Ray Diffraction (Xrd)
The XRD experimental data of the synthesized product agree with simulated diffractogram with mercury 3.8 software for Eu(H2O)6(C4O4)(HC4O4).H2O[15] complex with a shift involving the difference between the ionic rays between europium and Yttrium. Fig 1
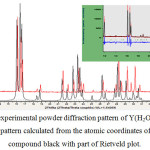 |
Figure 1: Parts of the experimental powder diffraction pattern of Y(H2O)6(C4O4)(HC4O4).H2O (red) and the powder pattern calculated from the atomic coordinates of the europium related compound black with part of Rietveld plot. Click here to View figure |
Indexation Des Diagrammes De Diffraction de Y(H2O)6(C4O4)(HC4O4).H2O
Indexing with DIVCOL 06 program leads in a monoclinic unit cell with parameters noted in Table 1 and figures of merit M (24) = 40.4, F (24) = 89.5 (0.0037, 73) revealing crystallization quality. The elemental cell in compound described by Jean-Francois Petit, and al is voluminous that we report. This justifies the shift noted in the diffractograms of Fig 2.
Table 1 : Cell parameters of M(H2O)6(C4O4)(HC4O4).H2O M = Eu, Y.
| a(Å) | b(Å) | c(Å) | α (°) | β (°) | γ(°) | V (Å3) | |
| Eu(H2O)6(C4O4)(HC4O4).H2O | 15.263(2) | 14.898(l) | 6.354(l) | 90 | 92.24(1) | 90 | 1444 |
| Y(H2O)6(C4O4)(HC4O4).H2O | 15.1987 | 14.8094 | 6.3209 | 90 | 92.271 | 90 | 1421.6 |
in Table 2 are collected indexation data of recorded diffractogram , showing the isotype of the two compounds.
 |
Table 2 : Indexing data of x-ray diffraction pattern for Y(H2O)6(C4O4)(HC4O4).H2O. Click here to View table |
![Figure 2: A packing plot of Eu(H2O)6(C4O4)(HC4O4).H2O[15] showing the intermolecular hydrogen-bonding scheme and tunnels encapsulates water molecules.](http://www.orientjchem.org/wp-content/uploads/2017/06/Vol33No3_Synt_Loui_fig2-150x150.jpg) |
Figure 2: A packing plot of Eu(H2O)6(C4O4)(HC4O4).H2O[15] showing the intermolecular hydrogen-bonding scheme and tunnels encapsulates water molecules. |
Thermal Analyses of Y(H2O)6(C4O4)(HC4O4).H2O
The thermal behaviours of Y(H2O)6(C4O4)(HC4O4).H2O show a three stage mass loss (FIG 3). The first stage between 90 and 113 correspond to elimination of the own water molecule, with a mass loss of 9.13, whereas in the second step the six water molecules coordinated to the metal atoms are emitted 115 and 260 with a mass loss of 19.33. In the last stage, decomposition of both squarate and mono hydrogen squarate ligands. The thermal decomposition product was identified as Y2O3. The final decomposition products of the complex was identified by powder RDX with the corresponding diffractogram obtained for the pure oxide
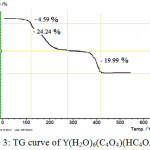 |
Figure 3: TG curve of Y(H2O)6(C4O4)(HC4O4).H2O Click here to View figure |
Our work aim lies in hybrid materials preparation, intercalating amines in their crystal lattices. In our approach, several procedures were tested. The only resulting complex is {(C2H10N2)1.5[Y(C4O4)3(H2O)4]}n [11]. To our knowledge, yttrium, squarate and amine complexes are less common. The structure of these materials with extensive networks including, the amine is specified from the viewpoint of properties of their analogue where the amine is not included in the network.
Catena-poly[sesqui(ethylenediammonium)[[tetraaquabis (squarato-қO) yttrium (III)]-μ-squarato-қ2O: O ‘]] [11] complex is characterized by a Two-dimensional crystalline structure. YO8 antiprisms formed constitute linear chains. The amines located between these chains participate, by hydrogen bonding, to interconnect these chains, thus forming layers which develop in parallel and stack along the axis c. They are located approximately 9° from each other. These layer arrangements reveal channels which develop parallel to the c axis and encapsulate protonated ethylenediamine (Fig 4).
![Figure 4 : Cristal lattice showing porosity of {(C2H10N2)1.5[Y(C4O4)3(H2O)4]}n [11].](http://www.orientjchem.org/wp-content/uploads/2017/06/Vol33No3_Synt_Loui_fig4-150x150.jpg) |
Figure 4 : Cristal lattice showing porosity of {(C2H10N2)1.5[Y(C4O4)3(H2O)4]}n [11]. |
The approach is to make varied the amine, by choosing templates with different carbon chains in their lengths, to favor the modifications of the properties of the material.
In Y(H2O)6(C4O4)(HC4O4).H2O complex, crystal lattice is characterized by presence of the tunnels along the c axis encapsulating water molecules (zeolite property). FIG 2 is reproduced from the crystallographic data file of Complex Eu(H2O)6(C4O4)(HC4O4).H2O [15] .
Conclusion
In the present work we have demonstrated that based on simple considerations concerning the nature of the metal atoms and coordination behaviour of the ligands the crystal structure of coordination polymers can be controlled to some extent. Whereas the occurrence of two-dimensional grids in these compounds was planned, the formation of the three-dimensional coordination network by interpenetration of these grids was not intended. However, compounds were obtained that contain channels in which additional water molecules are embedded. These channel water molecules can
-bipyridine metal squarates (Me=Mn, Fe, Co, Ni) showing the metal coordination in the hydrated compounds (left) and hypothetical structure of the fully dehydrated compounds (right).
reversibly be removed in a topotactic reaction. Surprisingly, also the removal of the water molecules which are coordinated to the metal atoms is reversible. The temperature of the steps and the energies involved depend on the nature of the metal atoms and can be understood on the basis of the slightly different crystal structures. The hydration and dehydration processes lead to continuous and significant changes of the optical properties of these compounds which can be attributed to a change of the coordination of the metal atoms.
Based on the structural data and the magnetic measurements a mechanism for the removal of the water molecules that are coordinated to the metal atoms is suggested. To prove this assumptions structural and spectroscopic investigations as well as theoretical calculations are planned. However, this will be the subject of further investigations.
Acknowledgments
We gratefully acknowledge the financial support by the State of Schleswig-Holstein. We are very thankful to Professor Dr. Wolfgang Bensch for helpful discussion, financial support and the facility to use his experimental equipment
Reference
- Chen, B.; Eddaoudi, M.; Hyde, S.T.; O’Keeffe, M.; Yaghi, O.M. Science. 2001, 291, 1021.
CrossRef - Cavellec, M.R.; Ferey, G. Solid State Sci. 2002, 4, 1221.
CrossRef - Murray, L. J.; Dinca, M.; Long, J. R. Hydrogen Chem. Soc. Rev. 2009, 38, 1294−1314.
CrossRef - Prasad, P.A.; Neeraj, S.; Natarajan, S., Rao, C.N.R. Chem.Commun. 2000, 1251
CrossRef - Dan, M,; Sivashankar, K,; Cheetham, A.K., Rao, C.N.R. J. Sol. Stat. Chem. 2003,174, 60–68
CrossRef - Erer, H.; Yesilel, O.Z.; Büyükgüngör, O. Polyhedron 2010, 29, 1163–1167
CrossRef - Forster, P.M.; Cheetham, A.K. Angew. Chem. Int. Ed. Engl. 2002, 41, 457.
CrossRef - Losier, P.; Zawarotko, M.J,Angew. Chem. Int. Ed. Engl. 1996, 35, 2779.
CrossRef - Eddaoudi, M,; Kim, J,, Rosi, N,; Vodak, D,; Wachter, J,; O’Keeffe, M,; Yaghi, O.M, Science 2002, 295, 469.
CrossRef - Castro, I,; Calatayud, M.L,; Sletten, J,; Lloret, F,; Julve, M, Inorg. Chim. Acta 1999, 287, 173.
CrossRef - Zenkhri, L,; Audebrand, N,; Bataille, T, Acta Cryst. 2011, 67, 529–530.
- XDR software, program with XRD équipement (Rennes).
- Boultif, A,; Louer, D,; J. APPL. CRYST. 2004, 37, 724-731.
CrossRef - Figure done using the MERCURY 3.8 freeware program from the Cambridge Crystallographic Data Centre.
- PETIT, J.F,; GLEIZES, A,; TROMBE. Inorg. Chim Acta. 1990, 167, 51-68.
CrossRef - J. Greve . Journal of Solid State Chemistry,2003, 175, 328–340 339.

This work is licensed under a Creative Commons Attribution 4.0 International License.









