Synthesis, Characterization and Antimicrobial Activity of New 2-Phenylquinoline-4(3H)-one Derivatives
Ali Hammadi Samir
Department of Chemistry, College of Education for Pure Science-Ibn Al-Haitham, Baghdad University, Baghdad, Iraq.
Corresponding Author E-mail: dr.mohammd08@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330344
Quinazolinones is an important chemical synthesis with different physiological significance and pharmacological utility. Reaction of anthranilic acid with benzoyl chloride in pyridine yielded 2-phenyl-4H-benzo[d][1,3]oxazin-4-one, which on condensation reaction with the tryptophan in glacial acetic acid afforded 3-(1H-indol-3-yl)-2-(4-oxo-2-phenylquinazolin-3(4H)-yl) propanoic acid (2). Treatment of compound (2) with thionyl chloride produced 3-(1H-indol-3-yl)-2-(4-oxo-2-phenylquinazolin-3(4H)-yl) propanoyl chloride (3). Condensation of compound (3) with hydrazine hydrate gave 3-(1H-indol-3-yl)-2-(4-oxo-2-phenylquinazolin-3(4H)-yl) propanehydrazide (4). Condensation of compound (4) with ethyl aceto acetate in glacial acetic acid yielded 3-(3-(1H-indol-3-yl)-1-(3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)-1-oxopropan-2-yl)-2-phenylquinazolin-4(3H)-one (5). The reaction of compound (4) with carbon disulfide and sodium hydroxide yielded 3-(2-(1H-indol-3-yl)-1-(5-mercapto-1,3,4-oxadiazol-2-yl) ethyl)-2-phenylquinazolin-4(3H)-one (6). Condensation of compound (6) with hydrazine hydrate afforded 2-hydrazinyl-5- (2-(1H-indol-3-yl) ethyl)-2-phenylquinazolin-4(3H)-one (7). Compound (8) 2-(3-(1H-indol-3-yl)-2-(4-oxo-2-phenylquinazolin-3(4H)-yl)propanoyl)-2,3-dihydrophthalazine-1,4-dione was synthesized by condensing compound (4) with phthalic anhydride in glacial acetic acid. When compound (4) was reacted with different aromatic aldehydes in abs. ethanol afforded different substituted (9a-d). Moreover, N-(1,5-dioxo-3-substituted-1,5-dihydrobenzo[e][1,3] oxazepin-4(3H)-yl)-3-(1H-indol-3-yl)-2-(4-oxo-2-phenylquinazolin-3(4H)-yl) propanamide (10a,b) were prepared from the cyclic condensation of Schiff bases compounds with phthalic anhydride. The structures of newly synthesized compounds were characterized by m.p., TLC, FTIR and 1H, 13C-NMR spectral analysis. The antimicrobial activity of the synthesized phenylquinazoline on two kinds of bacteria namely, staphylococcus aureous and Escherichia coli was investigated.
KEYWORDS:Phenylquinazoline; oxadiazole; triazole; pyrazole; oxazepin
Download this article as:| Copy the following to cite this article: Samir A. H. Synthesis, Characterization and Antimicrobial Activity of New 2-Phenylquinoline-4(3H)-one Derivatives. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Samir A. H. Synthesis, Characterization and Antimicrobial Activity of New 2-Phenylquinoline-4(3H)-one Derivatives. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33349 |
Introduction
Quinazolinone is one of the flourishing compounds in pharmaceutical fields. Quinazolines and its derivatives represent one of the most active classes of compounds. Medically it has been exhibited various activites as anti-bacterial 1,2 ,cytotoxic 3, analgesic , anti-inflammatory 4, anticonvusant 5,antitubercular 6,7 , anticancer8, and antioxidant9 , anti-HIV10,antitumour11,and anti-microbial 12. Quinazolinones derivatives were exhibit substantial in treating of leukemia than the common drugs13. Recent studies demonstrated the significant effect of quinazolinones derivatives via breast cancer in vitro study14-16.
Aim of the present study is to synthesis a new phenylquinazoline derivatives as antibacterial agents.
Material and Method
Chemistry
Melting points were determined using electrothermal melting point apparatus. A SHMADZU-FT-IR-8400 spectrometer was used to record the Infrared spectra atyrhemiss KBr disc. All 1H, 13C-NMR spectra measurement were made at Al-Albayt University, Jordan and Cairo University, Egypt on a Bruker 300 MHz and Avance III HD spectrometers using DMSO-d6 a solvent and TMS as internal reference. The progress of the reaction was monitored by TLC using aluminum silica gel plates. Grade solvent and reagents were purchased from commercial suppliers.
2-phenyl-4H-benzo[d][1,3] oxazin-4-one
0.01 mol. of C7H5OCl was gradually added to a 30 ml solution of 3.33×10-4 M of anthranilic acid in pyridine with continuous stirring at about 15˚C for 30 min. The formed product was separated while the reaction mixture stirred for 3 hours room temperature. Finally, a pale-yellow solid was yielded upon the addition of 15 ml. of 10% NaHCO3 was added to the solution. The formed precipitate was filtered off and washed with distilled water, dried and ethanol was used for recrystallization. Yield (68%), m.p. (118-120)˚C17.
Synthesis of 3-(1H-indol-3-yl)-2-(4-oxo-2-phenylquinazolin-3(4H)-yl) propanoic acid
(0.001mol.) of 2-phenyl-4H-benzo[d][1,3] oxazin-4-one was mixed with tryptophan in 15 ml of glacial acetic acid. The mixture was refluxed for (3-4) hr., followed by the addition of (25 ml) of Icy distilled water. The product was separated off by filtration and washed with distilled water, the dried. Recrystallization of the obtained product was carried out from ethanol. Yield (70%), m.p. (165-167)˚C18.
(1H-indol-3-yl)-2-(4-oxo-2-phenylquinazolin-3(4H)-yl)propanoyl chloride
A solution of compound 2 (0.01 mol.) and SOCl2 (2 ml.) in (20 ml.) benzene(dry) was refluxed for 4 hr. Crystals of the product were obtained by removing the excess of thionyl chloride and benzene under vacuum. yield (75%), m.p. (158-160)˚C 19.
3-(1H-indol-3-yl)-2-(4-oxo-2-phenylquinazolin-3(4H)-yl)propanehydrazide
0.01 mol. of compound 3 was mixed with 80% N2H4.H2O solution (0.5 ml., 5 mmol.) in dry benzene (10 ml.). After refluxing the mixture for five hours, the mixture was let to cool down to room temperature before removing the excess solvent and hydrazine under reduce pressure. The precipitate that obtained was washed with ether, and purified by recrystallized from ethanol. Yield (85%), m.p. (178-180)˚C 19.
(3-(1H-indol-3-yl)-1-(3-methyl-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)-1-oxopropan-2-yl)-phenylquinazolin-4(3H)-one
This compound was synthesized by refluxing (0.01 mol.) of compound 4 and ethyl acetoacetate (0.01 mol.) in 20 ml. glacial acetic acid for 6 hrs. After then, the mixture was cooled and 25 ml of icy distilled water was added, the reaction product (compound 5) was filtered out and dried. Yield (80%), m.p. (221-223)˚C 20.
3-(2-(1H-indol-3-yl)-1-(5-mercapto-1,3,4-oxadiazole-2-yl)ethyl)-2- phenylquinazolin-4(3H)-one
Twenty milliliters of ethanolic solution of (0.015 mol.) NaOH was mixed with (0.01 mol.) of compound 4 with continuous stirring for 15 min. Two milliliters of CS2 was then added before heating the mixture under reflux for 8 hrs. After concentrating the mixture, the reaction product was collected by acidification with dilute HCl solution. The product was then washed with water and recrystallized from ethanol. Yield (76%), m.p. (229-231)˚C 21.
2-hydrazinyl-5- (2-(1H-indol-3-yl) ethyl)-2-phenylquinazolin-4(3H)-one
To 20 ml of the ethanolic solution of compound 6 (0.01 mol), two milliliters of N2H4.H2O solution were added. After 7 hrs of reflux, the reaction mixture was left to cool down to room temperature. The pure yellow crystals of the product were obtained after removing the solvent under vacuum and recrystallization from ethanol. Yield (80%), m.p. (154-156)˚C 21.
2-(3-(1H-indol-3-yl)-2-(4-oxo-2-phenyl quinazolin-3(4H)-yl)propanoyl)-2,3-dihydrophthalazine-1,4-dione
This compound was obtained by refluxing a solution of an equimolar mixture (0.001 mol.) of compound 4 and phthalic anhydride in glacial acetic acid. After the addition of 20 ml. icy distilled water, the product was filtered and dried. Yield (80%), m.p. (121-123)˚C 22.
General Method for Synthesis of Schiff Bases 9a-d
An equimolar mixture (0.01 mol.) of and compound 4 with an appropriate aldehyde in (25 ml.) of absolute ethanol was refluxed in water bath for 8 hr after the addition of three drops of anhydrous CH3COOH. The formed product was collected by filtration and dried, after cooling the reaction mixture to room temperature23. The molecular formulas, melting points, and the yields of the synthesized compounds are presented in Table (1).
General Method for Synthesis of Oxazepines Compounds 10a,b
An equimolar mixture (0.01 mol.) of compounds 9a,b and phthalic anhydride was dissolved in (10 ml) of dry benzene and refluxed in a water bath for 10 hr. The solution was then cooled and treated with sodium bicarbonate to formed compound 10a,b. The reaction product i.e. 10a,b was separated by filtration, dried and recrystallized from ethanol24. Table (1) shows the data for compound (10a,b).
Biological Activity
The antimicrobial activity of the synthesized phenylquinazoline on two kinds of bacteria namely, staphylococcus aureous and Escherichia coli was investigated. The investigation was carried out via cup method with nutrient agar as a media for bacterial growth using 0.1ml of DMF as a solvent for all samples.The agar was scoop up from the sterilized cork borer cups and transferred into a bacterially inoculated petri dish. After then, a 0.1 ml aliquot of the solution of the investigated compound was introduced to each dish and the dishes were incubated for 24 hrs at 37°C.
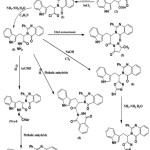 |
Scheme 1 |
Results and Discussion
Chemistry
The starting materials 2-amino-3-(1H-indol-3-yl) propanoic acid 2 , used in this work was prepared by refluxing of tryptophan with 2-phenyl-4H-benzo[d][1,3]oxazin-4-one (1) in glacial acetic acid. Analytical and spectral data were used to elucidate the structure of compound 2. The FTIR absorption spectrum of the compound displayed bands at 1685 cm-1 for carbonyl of acid, 2500-3200 cm-1 due to OH group of acid.
Compound 3 was obtained upon treatment of compound 2 with thionyl chloride in dry benzene. The presence of acyl chloride group was confirmed using its FTIR spectrum which display a strong absorption band at 1762 cm-1. The reaction of acyl chloride derivative 3 with hydrazide hydrate in refluxing ethanol gave the corresponding hydrazide derivative 4. The compound shows several bands at 1660 cm-1 which belong to carbonyl of amide, and at 3307, 3215 cm-1 for NH2 group. Moreover, its The 1H-NMR spectrum, fig. (1), shows characteristics chemical shifts, i.e. singlet signals at 5.67, 10.30, and 12.70 ppm which could be related to the protons of NH2 group, proton of NH group and the proton of OH group present in two tautomeric forms.
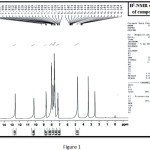 |
Figure 1 |
The triplet signal of proton for CH group at 4.30 ppm. Also showed the multiple signal at 6.92-8.68 ppm for aromatic protons of three benzene rings. The two peaks at at 164 and 167 in the 13C NMR spectrum of compound 4. fig. (2), indicate the presence of two resonating carbonyl groups of amide, while peaks at (127.40, 128.90) ppm are due to carbon of C=N group.
 |
Figure 2 |
Compound 4 was treated with ethyl acetoacetate in boiling glacial acetic acid to give compound 5. Bands at 1726, 1678 cm-1, 1637 cm-1, and 3309 cm-1 in the FTIR spectrum of compound 5 indicate the presence of CO amide, C=N, NH groups. The treatment of compound 4 with CS2 in presence of NaOH yields compound 6. The appearance of two peaks at (1616, 1600) cm-1 in the FTIR spectrum of the formed compound could be attributable to the presence of C=N of cyclic imine. Moreover, the 1H NMR spectrum of compound 6, fig. (3), exhibited two singlets at δ 11.69 and 11.71 which are specific for NH and SH protons, a triplet signal at δ 3.45 for proton of CH proton, a multiplet at δ 7.45-8.69 related to the aromatic-protons, a singlet at δ 8.69 for NH of cyclic tryptophan. Singlet signal at δ 4.38 for CH of cyclic tryptophan.
 |
Figure 3 |
Reaction of compound 6 with hydrazine hydrate in refluxing ethanol gave the corresponding compound 7 The FTIR spectrum of compound 7 confirmed the presence of intense absorption bands at 3329, 3105, 1662, and 1620 cm-1 due to amino, carbonyl amide and imine groups respectively. The 1H NMR spectrum of compound 7 ,fig.(4), showed two signals, triplet signal at δ 3.44 for CH proton and doublet signal at δ 2.68 for proton of CH2 group. A singlet signal at δ 10.45 for proton of NH, a multiple signal at δ 7.18-8.20 region related to aromatic protons, a signal at δ 8.40 for proton of NH of tryptophan.
 |
Figure 4 |
Compound 8 was synthesized by the condensation of compound 4 with phthalic anhydride in dry benzene. The chemical structure of this compound was determined by FTIR and 1H NMR spectroscopic techniques. The recorded infrared spectrum of the compound depicts the disappearance of νs and νas of NH2 group at (3317, 3165) cm-1, the appearance of two bands (1703, 1786) cm-1 for carbonyl group of cyclic amide, and a band rise at (1678) cm-1 due to carbonyl of amide. On the other hand, the proton-NMR spectrum of compound 8, fig. (5), shows many signals, a triplet at δ 3.59 and a doublet at δ 1.19 for CH and CH2 protons respectively, a singlet at δ 4.39 for the tryptophan five membered ring CH, two singlets at δ 11.76 and δ 12.01 for NH and OH protons in the two tautomeric forms, a multiple at δ 7.26-8.79 for the aromatic protons, and a singlet at δ 9.65 which corresponds to the tryptophan NH group.
 |
Figure 5 |
Compound 4 condensed easily with different aldehydes in ethanol containing piperidine at reflux temperature affording the corresponding Schiffʼs bases compounds 9a-d. The FTIR spectrum of compounds 9a-d showed disappearance of amino group and exhibited peak at region (1604-1649) cm-1 belonging to (C=N) group. The 1H NMR spectrum of compound 9a ,fig.(6),showed singlet signals at δ 10.57, 11.78, 11.95, and 12.13 corresponding to CH=N, NH of tryptophan, NH and OH protons in two tautomeric forms respectively. A multiple signals at δ 7.27-8.60 region owing to aromatic protons.The13CNMR spectrum of compound 9a, fig. (7), (DMSO): δ123.10-132.58(benzene rings), (127.24, 128.89) ppm due to two carbon of C=N groups. While (164.49-164.94) ppm due to two carbonyls of amide groups.
 |
Figure 6 |
 |
Figure 7 Click here to View figure |
 |
Figure 8 Click here to View figure |
 |
Figure 9 Click here to View figure |
Synthesis of compounds 10a,b was carried out via condensation of compounds 9a,b with phthalic anhydride in dry benzene. The purity structural formula of compounds 10a,b were confirmed by FTIR and 1H, 13C NMR spectroscopic techniques. The FTIR of compounds 10a,b showed a peak at (1664-1691) cm-1 belong to carbonyl of cyclic ester. The carbonyl absorption band of amide was observed at (1656-1691) cm-1. The 1H-NMR spectrum of compound 10 b, fig. (8), display two singles at δ 11.83 and 12.09 for NH and OH protons in the two tautomeric forms, singlet at δ 8.61 owing to protons of NHof tryptophan group, a multiple signal at δ (6.75-8.58) region attributed to aromatic protons. Two signals, triplet signal at 3.32 δ for CH proton and doublet signal at 2.86 δ for protons of CH2 group, singlet signals at 3.84 δ for CH of tryptophan. Singlet signal at 8.58 δ for (-CH-O-N) group. The 13C NMR spectrum of compound 10b, fig.(9), (DMSO): δ 28.23 (CH2), 123.01-132.33 (benzene rings), 164.40 (CO amide), 195.63 (CO of cyclic ester).
Table 1: Physical properties for Schiffʼs bases and 1,3-oxazepine compounds.
|
Comp. No. |
Ar |
Arˋ |
Molecular formula |
M. p. (˚C) |
Yield (%) |
|
9a |
4-BrC6H4– |
C32H25N5O2Br |
240-242 |
70 |
|
|
9b |
4,4-N,N(CH3)2C6H4– |
C34H31N6O2 |
220-222 |
75 |
|
|
9c |
4-ClC6H4– |
C32H25N5O2Cl |
217-219 |
80 |
|
|
9d |
C6H5– |
C32H26N2O2 |
198-200 |
78 |
|
|
10a |
4-BrC6H4– |
C40H28N5O5Br |
211-213 |
60 |
|
|
10b |
4,4-N,N(CH3)2C6H4– |
C42H34N6O5 |
206-208 |
70 |
Table 2: The FTIR absorption bands for Schiffʼs bases and 1,3-oxazepine compounds.
|
Comp. No |
N-H |
C-H ar. |
C-H ali. |
C=O amide |
C=O cyclic amide |
C=N |
C=C |
|
9a |
3192 |
3053 |
2988 |
1649 |
1623 |
1600 |
|
|
9b |
3186 |
3062 |
2970 |
1676 |
1635 |
1589 |
|
|
9c |
3227 |
3057 |
2960 |
1680 |
1604 |
1591 |
|
|
9d |
3221 |
3062 |
2983 |
1660 |
1649 |
1600 |
|
|
10a |
3223 |
3050 |
2966 |
1656 |
1693 |
1604 |
|
|
10b |
3180 |
3047 |
2970 |
1664 |
1691 |
1600 |
Biological Activity
In this study the antimicrobial activity of the synthesized phenylquinazoline on two kinds of bacteria namely, staphylococcus aureous and Escherichia coli was investigated. Table (3) shows the antibacterial activity of some synthesized compounds and the obtained zones .Results shows that compound 7 exhibit highly antibacterial activity against Escherichia coli and Staphylococcus aureus than other compounds.
Table 3 : Antibacterial activity of some synthezised compounds
|
Zone of inhibition |
(mm) | |
|
Comp. No |
Escherichia coli |
Staphylococcus aureus |
|
|
|
|
|
5 |
16 |
15 |
|
6 |
25 |
25 |
|
7 |
40 |
28 |
|
8 |
14 |
16 |
|
9 |
12 |
14 |
Conclusions
A series of newly phenylquinazoline compounds were successfully synthesized and characterized. Some of the newly compounds were tested for antibacterial activity which compound 7 exhibit highly antibacterial activity against Escherichia coli and Staphylococcus aureus than other compounds.
Acknowledgements
The author would like to thank the University of Baghdad for supporting this study and provide the grant for this study. We are also grateful to Dr.Zeinab M. AlRubaei for her contributions.
References
- A.A.Nagar, A. Patel, K.S. Rajesh ,K.R. Danao, and L.G.Rathi, Pharmagene 2013, 1(2),49-53 .
- J.Souad , B.L.Nabeel and M.B.Salah, Journal of Al-Nahrain University 2016,19 (1),1-12 .
CrossRef - N.K.Sinha ,A.J. Asnani ,B.R. Dravyakar ,Asain journal of pharmaceutical and clinical research 2013,6(3) .
- W.Dan and G.Feng , Chemistry Central Journal 2013,7(95),1-15 .
- D.Mukherjee ,A. Mukhopadhyay ,K.B. Shridhara, A.M.Shridhara, K.S.Rao , International Journal of Pharmacy and Pharmaceutical Sciences 2014 , 6( 5),567-571 .
- M.K.Srivastav and S.M.Shantakumar, Chemical Science Transaction. 2013, 2(3): 1056-1062 .
- O.H.Abid and A.H.Ahmed , Inter J Appl Nat Sci. 2013, 2,11–20 .
- A.Shetha and I.A.Wijdan , J Chem Pharm Res. 2013,5,: 42–45 .
- Zaranappa et al. , Int J Chem Tech Res. 2012,4, 1527–1533 .
- B.Pati . and S.Banerjee , JAdv Pharm Edu Res. 2013, 3, 136–151 .
- L.Wei ,Z. Xiaotian , C.Yang , G.Shunmin ,T. Feifei Ba,Wei , Y.Chao ,W. Mingping ,L.Yu , S.Yunlong ,Z.Ju ,Z.Weicheng , Z.Youjun ,Z.Feng ,G. Hao ,Z.Canhui ,Tetrahedron 2016,72 (23), 3185–3192.
- T.Shweta, M.Vikas , S.Vasudha , S.Pushplata , S.Manjul ,Asian Journal of Pharmaceutical and Clinical Research 2012 , 5( 1),98-100 .
- A.V.Danilov ,Clinical Therapy 2013, 35,1258–1270 .
CrossRef - M.F.Ahmed and M.Youns, Archiv der Pharmazie. 2013,346,610–617 .
CrossRef - D.Kumar ,G.Mariappan ,A.Husain ,J.Monga and S.Kumar, Arabian J. Chem. 2014,10,1016.
- F.L.Faraj et al. , Sci. Wld J. 2014, 10,1155.
- D.Gor,P. Patel, M.Shah, and S.Patel, Der Pharma Chemical 2012,4(2),626-628.
- K.Faghihi, M.Nourbakhsh, and M.Hajibeygi, Journal of Saudi Chemical Society 2011, Inpress,xxx,:xxx-xxx.
- M.S. Fouad, I.Redha, Al-Bayati, and Araa Al-Juboori. , Al-Mustansiriya Journal of Science 2006,17(3),15-26.
- A.H.Mekky, ,ph.D.Thesis ,college of Science ,University of Al-Mustansiriya,2015.
- S.Cao , X.Qian , G.Song. ,Q. Huang, Journal of Fluorine Chem.2002, 117(1),63-66.
CrossRef - I.K.Jassim, S.A.Jabbar and A.Waaled , Karbala Journal of Parmaceutical Science 2015, 10, 102.
- M. Noolvi ,S.Agrawal , H.Patel ,A.Badiger ,M.Gaba and A.Zambre ,.Arabian Journal of Chemistry 2014,7(2),219-226.
CrossRef - R.W.Adam and E.H. Zimam , .Karbala Journal of Pharmaceutical Science 2014,7,195-207

This work is licensed under a Creative Commons Attribution 4.0 International License.









