Studies of Electric Dipole Moment and Magnetic Dipole Moment of Substituted Benzothiazolyl and Benzimidazolyl Derivatives
Ankush W. Wakode, Archana S. Burghate and Shrikant A. Wadhal
Department of Chemistry, Shri Shivaji Science College, Amravati, 444603, M. S. India.
Corresponding Author E-mail: ankushwakode@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330319
Refractive indices of benzothiazolyl and benzimidazolyl derivatives with different concentration in binary liquid mixture such as acetone-water, Dioxane-water & DMSO–water at 35± 0.1˚C were measured by Abbe’s refractometer. The data obtained was utilized to calculate molar polarization & electric dipole moment which explain solute-solvent interactions. The magnetic susceptibility and magnetic dipole moment calculated by using Gouy’s method.
KEYWORDS:Electric dipole moment; Effective magnetic moment; Magnetic susceptibility
Download this article as:| Copy the following to cite this article: Wakode A. W, Burghate A. S, Wadhal S. A. Studies of Electric Dipole Moment and Magnetic Dipole Moment of Substituted Benzothiazolyl and Benzimidazolyl Derivatives. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Wakode A. W, Burghate A. S, Wadhal S. A. Studies of Electric Dipole Moment and Magnetic Dipole Moment of Substituted Benzothiazolyl and Benzimidazolyl Derivatives. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=32934 |
Introduction
Benzothiazolyl and benzimidazolyl substituted derivatives are the heterocyclic compounds with wide application in pharmaceutical chemistry and have wide range of biological applicability1. In the present work, Benzothiazolyl and benzimidazolyl derivatives are widely found in bioorganic and medicinal chemistry with applications in drug discovery and development for treatment such as autoimmune and inflammatory diseases in the prevention of solid organ transplant rejection, epilepsy2, antitumor, antiviral3, anticonvulsant4, antiallergic, antitumor5, neuroprotective and immunosuppressive properties. The studies of refractive indices are being increasingly used as tools6-7 for investigation of the physical properties of pure components and the nature of inter molecular interaction between the liquid mixture constituents8 Refractive index measurements in combination with density, boiling point, melting point and other analytical data have wide applicability in chemical analysis an industry9 Many researchers studied Schiff’s bases with the determination of physical parameter10. Schiff’s bases uses are some of the most widely used organic compound they are use as pigment and dyes, catalysts, intermediates in organic synthesis, and as polymer stabilizers. Analytical performance of refractometry in quantitative Estimation of isotopic concentration of heavy water in nuclear reactor11.Many workers12-13 studied physical parameters of organic compounds binary liquid mixtures to judge molecular interactions.
Measurement of magnetic properties has been used to characterize a wide range of systems from oxygen, metallic alloys, solid state materials, and coordination complexes containing metals. Most organic and main group element compounds have all the electrons paired and these are diamagnetic molecules with very small magnetic moments. All materials show some reaction to a magnetic field though is the case of conventionally non-magnetic materials the reaction will be very weak a powerful electromagnet and sensitive measuring instrument are needed to demonstrate these weak reactions. The magnetic properties like magnetic susceptibility, magnetic moment can be studied from Gouy method. In present study electric dipole moment is determined from refractometric study and magnetic dipole moment by Gouy’s method.
Experimental
Acetone-water, Dioxane-water and DMSO–water mixture as well as solutions of Benzothiazolyl and benzimidazolyl substituted derivatives of different concentration were prepared. All weighing was made on contech electronic balance ( ±0.001g). The accuracy of density measurements was within 0.1 kg m-3. The refractive indices of solvent mixture and solutions were measured by Abbes’ refractometer was within (± 0.001). The densities of the solutions were determined by pyknometer. The temperature of prism box was maintained at 36˚C. Initially, the refractometer was calibrated with glass piece (n = 1.5220) provided with the instrument. The magnetic measurement was carried out by Gouy’s method and CuSO4. 5H2O was used as calibrant.
The present work deals with the study of molar refraction and polarizability constant of Benzothiazolyl and benzimidazolyl substituted derivatives in different concentration of binary mixture at 307K (35˚C) . The data obtained have been used to compute intermolecular interaction. The selected compounds for the study may be summarised as
| Name of compounds | Symbol | Molecular formula |
| 1-Benzothiazole-2-yl-[1,2]diazetidin-3-one |
1a |
C9H7N3SO |
| (1-Benzothiazol-2-yl-[1,2]diazetidine-3-ylidene)-4(phenyl-thiazole-2-yl)-amine |
1b |
C18H13N5S2 |
| 1-Benzothiazol-2-l-[1,2]diazetidine-3-ylidene)-phenyl-amine |
1c |
C15H12N4S |
| 1,2-Dihydro-benzo[4,5]imidazo[2,1-c][1,2,4]triazin-3-one |
2a |
C9H8N4O |
| (1,2-Dihydro-benz[4,5]imidazo[2,1-c][1,2,4]triazin-3-ylidene)-(4-phenyl-thiazole-2-yl)-amine |
2b |
C18H14N6S |
The various equations used in electric and magnetic dipole moment study may be reported as
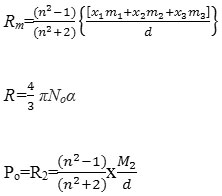
µ=0.128×10-18x(P2T)1/2 debye
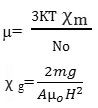
µeff =2.828(χ AT)½
Results and Discussion
Refractive index is an important physical parameter of liquids. The potential of refractometry depends on sensitivity and time of measuring. In the present study refractive index of solvent-water mixture and Benzothiazolyl and benzimidazolyl substituted derivatives in solvent water mixture were determined. Molar refraction and polarizability electric dipole moment were determined for variation in concentration of compounds in 75% solvent-water mixture. The respective polarizability and electric dipole moment may be presented as in table-1.
Table 1.1: Molar Polarization and electric dipole moment w.r.t. concentration variation
System: 1a in 75% Dioxane-water Temperature: 35± 0.1 ˚C
|
Sr.No. |
CONC. (M) |
αx10-23 |
Po |
µ (esu-cm) |
|
1 |
0.01 |
0.006873 |
0.173274 |
9.35088E-19 |
|
2 |
0.005 |
0.008556 |
0.215706 |
1.04332E-18 |
|
3 |
0.0025 |
0.01016 |
0.256147 |
1.13692E-18 |
|
4 |
0.00125 |
0.01201 |
0.302809 |
1.23615E-18 |
|
5 |
0.000625 |
0.014162 |
0.35705 |
1.3423E-18 |
Table 1.2: Molar Polarization and electric dipole moment w.r.t. concentration variation
System: 2a in 75% Dioxane-water Temperature: 35± 0.1 ˚C
|
Sr.No. |
CONC. (M) |
αx10-23 |
Po |
µ (esu-cm) |
|
1 |
0.01 |
0.012308 |
0.310304 |
1.25135E-18 |
|
2 |
0.005 |
0.013684 |
0.344995 |
1.31945E-18 |
|
3 |
0.0025 |
0.01379 |
0.347686 |
1.32458E-18 |
|
4 |
0.00125 |
0.013945 |
0.351578 |
1.33197E-18 |
|
5 |
0.000625 |
0.014306 |
0.360696 |
1.34914E-18 |
Table 1.3: Molar Polarization and electric dipole moment w.r.t. concentration variation
System: 1c in 75% Dioxane-water Temperature: 35± 0.1 ˚C
|
Sr.No. |
CONC. (M) |
αx10-23 |
Po |
µ (esu-cm) |
|
1 |
0.01 |
0.00769 |
0.193885 |
9.8914E-19 |
|
2 |
0.005 |
0.00774 |
0.19514 |
9.92335E-19 |
|
3 |
0.0025 |
0.007785 |
0.196275 |
9.95218E-19 |
|
4 |
0.00125 |
0.008731 |
0.220125 |
1.05395E-18 |
|
5 |
0.000625 |
0.010042 |
0.253191 |
1.13034E-18 |
Table 1.4: Molar Polarization and electric dipole moment w.r.t. concentration variation
System: 1a in 75% Acetone-water Temperature: 35± 0.1 ˚C
|
Sr.No. |
CONC. (M) |
αx10-23 |
Po |
µ (esu-cm) |
|
1 |
0.01 |
0.076164 |
1.92025 |
3.11289E-18 |
|
2 |
0.005 |
0.105864 |
2.669065 |
3.66999E-18 |
|
3 |
0.0025 |
0.106959 |
2.696664 |
3.68892E-18 |
|
4 |
0.00125 |
0.108923 |
2.746186 |
3.72263E-18 |
|
5 |
0.000625 |
0.10999 |
2.773091 |
3.74083E-18 |
Table 1.5: Molar Polarization and electric dipole moment w.r.t. concentration variation
System: 2a in 75% Acetone-water Temperature: 35± 0.1 ˚C
|
Sr.No. |
CONC. (M) |
αx10-23 |
Po |
µ (esu-cm) |
|
1 |
0.01 |
0.155084 |
3.90999 |
4.44194E-18 |
|
2 |
0.005 |
0.164555 |
4.148791 |
4.57558E-18 |
|
3 |
0.0025 |
0.161438 |
4.070202 |
4.53204E-18 |
|
4 |
0.00125 |
0.18447 |
4.650881 |
4.84454E-18 |
|
5 |
0.000625 |
0.192787 |
4.860568 |
4.95255E-18 |
Table 1.6: Molar Polarization and electric dipole moment w.r.t. concentration variation
System: 1c in 75% Acetone-water Temperature: 35± 0.1 ˚C
|
Sr.No. |
CONC. (M) |
αx10-23 |
Po |
µ (esu-cm) |
|
1 |
0.01 |
0.102004 |
2.57174 |
3.60246E-18 |
|
2 |
0.005 |
0.105057 |
2.648719 |
3.65598E-18 |
|
3 |
0.0025 |
0.107465 |
2.709412 |
3.69762E-18 |
|
4 |
0.00125 |
0.108666 |
2.739689 |
3.71823E-18 |
|
5 |
0.000625 |
0.111973 |
2.823069 |
3.77438E-18 |
Table 1.7: Molar Polarization and electric dipole moment w.r.t. concentration variation
System: 1a in 75% DMSO-water Temperature: 35± 0.1 ˚C
|
Sr.No. |
CONC. (M) |
αx10-23 |
Po |
µ (esu-cm) |
|
1 |
0.01 |
0.03246 |
0.818372 |
2.03E-18 |
|
2 |
0.005 |
0.039128 |
0.986494 |
2.23E-18 |
|
3 |
0.0025 |
0.04352 |
1.097226 |
2.35E-18 |
|
4 |
0.00125 |
0.048497 |
1.222721 |
2.48E-18 |
|
5 |
0.000625 |
0.051282 |
1.292926 |
2.55E-18 |
Table 1.8: Molar Polarization and electric dipole moment w.r.t. concentration variation
System: 2a in 75% DMSO-water Temperature: 35± 0.1 ˚C
|
Sr.No. |
CONC. (M) |
αx10-23 |
Po |
µ (esu-cm) |
|
1 |
0.01 |
0.00096 |
0.024211 |
3.5E-19 |
|
2 |
0.005 |
0.002764 |
0.069698 |
5.93E-19 |
|
3 |
0.0025 |
0.00398 |
0.100349 |
7.12E-19 |
|
4 |
0.00125 |
0.006274 |
0.158175 |
8.93E-19 |
|
5 |
0.000625 |
0.007388 |
0.186279 |
9.7E-19 |
Table 1.9: Molar Polarization and electric dipole moment w.r.t. concentration variation
System: 1c in 75% DMSO-water Temperature: 35± 0.1 ˚C
|
Sr.No. |
CONC. (M) |
αx10-23 |
Po |
µ (esu-cm) |
|
1 |
0.01 |
0.041096 |
1.036116 |
2.29E-18 |
|
2 |
0.005 |
0.041881 |
1.055898 |
2.31E-18 |
|
3 |
0.0025 |
0.050713 |
1.278585 |
2.54E-18 |
|
4 |
0.00125 |
0.050866 |
1.28244 |
2.54E-18 |
|
5 |
0.000625 |
0.057625 |
1.452841 |
2.71E-18 |
In the present investigation, with decrease in concentration of compounds, polarizability as well as electric dipole moment increases in order of solvent mixture Acetone-water > DMSO-water > Dioxan-water from the results it may be predicted that for the compounds 1a, 2a,1c in various solvents, results in increase in polarity this results due to solvent-solvent interaction is greater than solute-solvent interaction.
The some compounds were implemented for magnetic properties study by using Gouy method. Solids exhibiting only electronic polarizability are elemental solid dielectrics. Solids which manifest electronic and ionic polarizabilities are ionic nonpolar dielectrics and solids which possess orientational, electronic and ionic polarizabilities are polar solids.
Table 2
|
Sr No. |
Name of System |
Mol. Wt. | χm ( m3·mol−1) |
µeff (BM) |
|
1 |
2a |
188.19 | 0.020557 | 0.223193 |
|
2 |
1b |
363.47 | 0.045376 | 0.331599 |
|
3 |
1c |
280.36 | 0.035 | 0.291231 |
|
4 |
1a |
205.25 | 0.025624 | 0.249184 |
|
5 |
2b |
346.42 | 0.043247 | 0.323728 |
|
6 |
1e |
325.36 | 0.040618 | 0.313734 |
In the present study for the compounds 1a,1b, 1c, 2a, 2b shows specific value for magnetic dipole moment. The existence of magnetic property in 1b,1c, and 2b may be due to dipole formation at aliphatic ring across >C=N- group where as in case of 1a and 2a, the dipole formation may be at carbonyl group . These dipoles may be formed due to electronegativity difference in the binding atoms. The observed values of magnetic susceptibility and magnetic dipole moment in the substituted benzothiazolyl and benzimidazolyl compounds predicts that their magnetic nature may be due to spin and orbital motion of electrons14.
Acknowledgement
The authors would like to thanks authorities of Shri Shivaji Science College, Amravati University, Amravati for providing necessary facilities.
References
- Wakode, A. W.; Burghate, A. S.; Wadhal, S. A. Indo American Journal of Pharmaceutical Research . 2014, 4, 5010-5016.
- Hangirgekar, S. P.; Shirodkar, S. G. Indo American Journal of Pharmaceutical Research. 2011, 1, 153-157.
- Kawde, P.R.; Deohate, P. P.; Berad B. N. Indian Journal of Chemistry. 2015,54B, 833-836.
- Singh, M.; Gangwar, M.; Nath, G.; Singh, S. K. Indian Journal of Chemistry. 2014,52, 1062-1070.
- Baheti, K. G.; Jadhav, J. S.; Suryavanshi, A. T. Indian Journal of Chemistry. 2005,44B, 834-837.
- Nain, A. K.; Chandra, P.; Pandey, J. D.; Gopal, S. Journal of Chemical & Engineering Data. 2008, 53, 2654-2665.
CrossRef - Nain, A. K.. Fluid Phase Equilibria, 2008, 265, 56.
CrossRef - Ansari, N. H. Open Journal of Physical chemistry, 2014, 4, 1-5.
CrossRef - Ubarhande, S. S.; Burghate, A. S.; Berad, B. N.; Turak, J. D. Rasayan J. Chem. 2011, 4, 585-587.
- Talegaonkar,* R. S.; Burghate, A. S.; Wadhal, S. A. Indian J. Heterocyclic Chem. Vol. 2011, 20, 413-414.
- Dhole, K.; Ghosh, S.; Datta, A.; Tripathy, M. K.; Bose, H. Barc Newsletter, 2011, 320, 7-11.
- Wakode, A. W.; Burghate, A. S.; Wadhal, S. A. Rasayan J. Chem. 2016,4, 386-392.
- Talegaonkar, R. S.; Burghate, A. S; Wadhal, S. A. Orient. J. Chem. 2011, 27, 1285-1288.
- Pillai S. O. Solid State Physics. Seventh edition, New Age International (P) Limited, Publication. 2015.

This work is licensed under a Creative Commons Attribution 4.0 International License.









