Optical and Antibacterial Studies of Zinc Magnesium Oxide Nanocomposite
C. R. Indulal1, R. Biju2, Deepak Nand2 and R. Raveendran2
1Department of Physics, S.G. College, Kottarakara, Kerala, India.
2Nanoscience Research laboratory, S.N. College, Kollam, Kerala, India.
Coorespnding Author E-mail: ltdrindulal@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330359
Through the microwave assisted fast synthesis method, Zinc Magnesium Oxide nanocomposite was prepared. Zinc Magnesium Oxide nanocomposite heated at 500ºC was used for further structural, optical and antibacterial studies. The Debye Scherrer equation was used to calculate the average particle size of the nanocomposite. The optical characterization of the oxide nanocomposite was carried out by UV analysis. From the analysis of the absorption spectra, the optical direct band gap of the Zinc Magnesium Oxide nanocomposite was calculated and the result was compared with Zinc Oxide and Magnesium Oxide nanocomposites. Antibacterial studies were done in detail.
KEYWORDS:Nanocomposites; XRD; Optical band gap; Antibacterial studies
Download this article as:| Copy the following to cite this article: Indulal C. R, Biju R, Nand D, Raveendran R. Optical and Antibacterial Studies of Zinc Magnesium Oxide Nanocomposite. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Indulal C. R, Biju R, Nand D, Raveendran R. Optical and Antibacterial Studies of Zinc Magnesium Oxide Nanocomposite. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=34065 |
Introduction
Nanooxide materials with large surface to volume ratio acquire unique magnetic, electronic, optical, antibacterial properties and potential applications. In the early 1950s, scientists had already started the research on Zinc related materials as antibacterial agents1. In the areas related with textile industries, water disinfection, medicine, food packaging etc. these antibacterial agents have a major role. Instead of organic compounds, inorganic disinfectants like metal oxide nanocomposites have several advantages, including their non toxicity to the human body. Zinc oxide is a wide band gap semiconducting material with a lot of applications including light emitting diodes, piezoelectric transducers, photocatalysts etc2-4.
Nanomaterials exhibit large surface to volume ratio and most of their properties are selectively controlled by engineering the size, morphology and composition. Such nano crystalline metal oxides exhibiting this large surface area can be applied to devices including sensors for which a better surface effect is required. These new nanomaterials can have enhanced properties from their parent bulk materials5. The nano metal oxides nanocomposites exhibit exceptional UV absorbing ability, high stability at high temperatures and reactivity as catalyst6-7.
Experimental Procedure
Zinc Magnesium Oxide nanocomposite was prepared by microwave assisted fast synthesis method using analytical grade Magnesium Nitrate and Zinc Nitrate as the reagents. When compared with other methods, this method has revealed several advantages. It was already reported that molecules undergo excitations due to electromagnetic radiations. By converting microwave radiation into heat energy with high efficiency, superheating becomes possible at ambient pressure. Zinc Nitrate, Magnesium Nitrate and Sodium hydroxide were used as starting materials. Citric acid was used as stabilizer. Aqueous solutions of 0.1M Zinc Nitrate, 0.1M Magnesium Nitrate and 0.5M Sodium hydroxide were mixed drop wise and stirred simultaneously into a beaker containing aqueous solution of 0.02M Citric acid. The process was carried out in few minutes. Then the beaker was kept in a microwave oven. The stabilizer is used to prevent growth/agglomeration of the particles.
Characterization
XRD studies are ideal for the of nanocomposites size determination of powder samples. The XRD patterns of the powdered samples were recorded using XPERT-PRO powder diffractometer using Cu- Ka radiation in the 2q range 10° to 80° at 30mA, 40kV. Based on the line broadening, few techniques involving Scherrer equation, integral breadth analysis or Hall-Williamson approach and Fourier method of Warren-Averbach have been developed8-10.The UV spectrum were recorded using Shimadzu UV-2550 UV visible spectrophoto meter. Antibacterial activity of the sample was carried out using diffusion disk method.
Results and Discussions
XRD Studies
The nano crystalline nature of Zinc Magnesium Oxide is verified using XRD analysis. There is a definite line broadening of the XRD peaks which indicates the synthesized materials consist of particles in nanometer scale. The peak intensity, position and full width at half maximum data are obtained from the XRD pattern. The nanoparticle sizes are calculated using Debye-Scherrer formula, d = 0.9λ/bCosθ11, where 0.9 is the Scherrer constant, β is the full width at half maximum of XRD lines, θ is Bragg diffraction angle and wavelength of X-rays, λ = 1.54060 [Å]. The XRD pattern of Zinc Oxide (ZnO), Magnesium Oxide (MgO) and Zinc Magnesium Oxide (ZnMgO) heated at 500ºC are shown in fig 1A, 1B and 1C respectively. The average particle size for Zinc Oxide is 19nm, Magnesium Oxide is 7nm and Zinc Magnesium Oxide is 46nm. The micro straining of crystal structures due to various defects such as dislocations and twinning are the major reasons behind the broadening of the peaks in the XRD pattern. These defects are supposed to be linked with chemically synthesized nanocomposites. When the crystals suddenly grows during chemical reaction, the legands do not get enough time to diffuse to an energetically positive site resulting in crystal defects12.The XRD peaks of Zinc Oxide, Magnesium Oxide and Zinc Magnesium Oxide confirms that they are almost free from impurities.
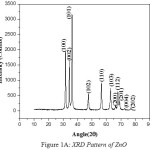 |
Figure 1a: XRD Pattern of ZnO |
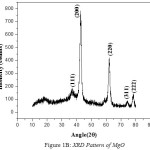 |
Figure 1b: XRD Pattern of MgO |
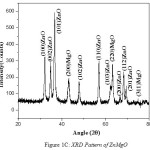 |
Figure 1c: XRD Pattern of ZnMgO |
UV Spectral Studies
The UV spectra of Zinc Oxide, Magnesium Oxide and Zinc Magnesium Oxide heated at 500ºC taken in the wavelength range of 210 to 870 nm are shown in fig 2A, 2B and 2C respectively. The optical band gap details of the nanocomposites can be directly calculated using UV absorption spectra. The decrease in absorbance with increase in wavelength from the UV spectra is due to the presence of optical band gap in the nanomaterials. Using Tauc’s relation, the optical direct band gap of the nanomaterials can be calculated13. The value of optical direct band gap of Zinc Oxide is 3.03eV, Magnesium Oxide is 3.2eV and Zinc Magnesium Oxide nano composite is 3.29eV and are depicted in the figures 3A, 3B and 3C respectively. So compared to their single counter parts, the nano composites have a considerably large band gap value.
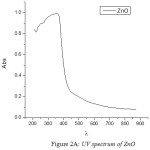 |
Figure 2a: UV spectrum of ZnO |
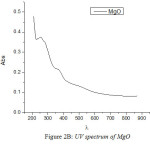 |
Figure 2b: UV spectrum of MgO |
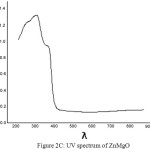 |
Figure 2c: UV spectrum of ZnMgO |
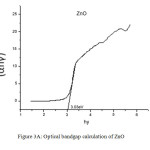 |
Figure 3a: Optical bandgap calculation of ZnO |
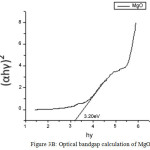 |
Figure 3b: Optical bandgap calculation of MgO |
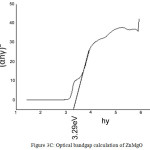 |
Figure 3c: Optical bandgap calculation of ZnMgO |
Antibacterial Studies
In order to investigate the antibacterial ability of Zinc Magnesium Oxide nanocomposite, the diffusion disk method was employed. Staphylococcus aureus, Vibrio Cholerae, Escherichia coli and Bacillus cereus were selected as test strains14. The inhibition zone diameters of Staphylococcus aureus, Vibrio Cholerae, Escherichia coli and Bacillus cereus found to be 14, 20, 15 and 0 mm respectively (Fig4A). Zinc Magnesium Oxide nanocomposite is found to be an efficient antibacterial agent towards Vibrio Cholerae. However it shows very poor activity towards Bacillus cereus.
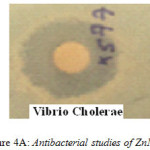 |
Figure 4a: Antibacterial studies of ZnMgO |
Conclusions
Nanocomposite of Zinc Magnesium Oxide was prepared by microwave assisted fast synthesis method. Mean particle sizes obtained from XRD studies are 46nm for Zinc Magnesium Oxide, 19nm for Zinc Oxide and 7nm for magnesium oxide and are in the nano meter scale. Particle size of nanocomposite is greater than their single counter parts. The value of direct band gap of Zinc Magnesium Oxide nanocomposite is 3.29eV which is greater than Zinc oxide or Magnesium oxide nanoparticles. It can be concluded that highly reactive metal oxide nanocomposites of Zinc Magnesium Oxide exhibit exceptional antibacterial activity against both gram-positive and gram-negative bacteria except Bacillus cereus.
References
- Bartels, H.A.; The effect of eugenol and oil of cloves on the growth of micro.overddot.organisms. Am J Orthod Oral Surg. 1947,33, 458-465.
CrossRef - Bao, J; Zimmler, M A; Capasso, F; Broadband ZnO single-nanowire light-emitting diode Nano Letters. 2006, 6, 1719-1722.
CrossRef - Bai, X. D.; Gao, P. X.; Wang, Z. L.; Wang, E. G.; Dual-mode mechanical resonance of individual ZnO nanobelts, Applied Physics Letters. 2003, 82, 4806-4808.
CrossRef - Lin, H. F.; Liao, S. C.; Hung, S. W.; The dc thermal plasma synthesis of ZnO nanocomposites for visible- light photocatalyst, Journal of Photochemistry and Photobiology A. 2005,174, 82-87.
CrossRef - Kenneth J. Klabunde; Cathymohs, Department of Chemistry, Kansas state university, Manhanttan Kansas second Edition, 1998.
- Tsai, M. S.;Mater. Sci. Eng. B 2004,110, 132-134.
CrossRef - Mori; Wang, Y. R.; Drennan; J Solid State Ionics. 2004, 175, 641-649.
CrossRef - Scherrer, P; Math. Phys. K. 1918, 1, 187-188.
- Williamson, G. K.; Hall, W. H.; Acta Metall. 1953, 1, 22-31.
CrossRef - Warren, B. E.; Averbach, B. L.; J. Appl. Phys. 1950, 21, 595-599.
CrossRef - Cullity, B. D.; Elements of X-ray diffraction, Addison – wesley publishing company Inc. California, 1970, 102.
- Warad, H. C.; Ghosh, S C; Hemtanon; Sci. Technol. Adv. Mater. 2005, 6, 296-301.
CrossRef - Mohanta, D.;Nath, S. S.;Mishra, N. C.; Choudhury, A.; Bull. Mater. Sci. 2003, 26, 289-294.
CrossRef - Shalumon, K. T.; International Journal of Biological Macromolecules 2011, 49, 247-254.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









