Impacts of Nickel Nanoparticles on Grow Characteristics, Photosynthetic Pigment Content and Antioxidant Activity of Coriandrum Sativum L.
Abdolhossein Miri1, Elham Sadat Shakib1, Omolbanin Ebrahimi1 and Javad Sharifi-Rad2
1Department of Pharmacognosy, Faculty of Pharmacy, Zabol University of Medical Sciences, Zabol, Iran.
2Phytochemistry Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Corresponding Author E-mail: javad.sharifirad@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330329
Medicinal plants consumption is used in various countries for treatment disease and food complementary. Nanotechnology is one of developing sciences in the world. The research performed in previous studies showed that properties of medicinal plants could be strongly affected by nanoparticles that have polluted the environment. In this study, we investigated effects of nickel nanoparticles on root and shoot elongation, relative water content (RWC), photosynthetic pigment, total ash, antioxidant activity of Corianderum sativum L. The results showed that nickel nanoparticle decreased the RWC, root and shoot elongation, the content of photosynthetic pigments, %total ash. In addition, although the nanoparticles decreased the antioxidant activity. The results of this study have shown that, nickel nanoparticles have toxic effects on C. sativum L plant. This research could be a turning point in the field of nanotechnology research in medicinal plants. Other researchers can survey by themselves any aspects of the study with more focusing on molecular mechanisms.
KEYWORDS:Nickel nanoparticles; Coriandrum sativum; antioxidant activity; RWC
Download this article as:| Copy the following to cite this article: Miri A, Shakib E. S, Ebrahimi O, Sharifi-Rad J. Impacts of Nickel Nanoparticles on Grow Characteristics, Photosynthetic Pigment Content and Antioxidant Activity of Coriandrum Sativum L. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Miri A, Shakib E. S, Ebrahimi O, Sharifi-Rad J. Impacts of Nickel Nanoparticles on Grow Characteristics, Photosynthetic Pigment Content and Antioxidant Activity of Coriandrum Sativum L. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33680 |
Introduction
Nanotechnology is an attractive research field because the use of nanoparticles due to particular physicochemical characteristics are increasing in the various sciences(1). Nanoparticles have much applied in agriculture, industry, pharmaceutical and medicine sciences (2, 3). In spite of the extensive growth of nanotechnology and nanomaterials through the last twenty years, the recent focus has been turned on to the potential toxicological effects on animals, humans and the environment through the exposure of metal nanomaterials (4).
Nanotechnology is a new science founded ways to diverse industries and several fields of researches. Life sciences are no exception in this regard (5-8). Materials with at least one dimension between 1-100 nm are called nanoparticles (9). The small size and large surface to volume ratio give them very unique and extraordinary properties which have caused significant progresses in different majors including food sciences, biomedical sciences, gene therapy, drug delivery, and cell targeting. Usage of nanoparticles has had remarkable effects on plant development and growth (10, 11). Although, there are many beneficial properties for nanoparticles on plant growth, the adverse effects should be also discussed.
Nanosized nickel oxide (nNiO) possesses many unique properties compared to its bulk counterpart and is extensively used as catalyzer, battery electrode, electrochromic films, sensors magnetic materials and diesel–fuel additive (12). The risks of inhalation exposure to NiO nanoparticles has been reported in mammal as determined with in vitro assays (12).
Coriandrum sativum also known as cilantro, Chinese parsley or dhania is an annual herb in the Apiaceae. It is a soft, hairless plant growing to 50 cm. The leaves are uneven in shape, broadly lobed at the base of the plant and slender. The flowers are borne in small umbels, white asymmetrical with the petals pointing away. In the Indian traditional medicine, a coriander is used in disorders of digestive, respiratory and urinary system, as it has diaphoretic, diuretic, carminative and stimulant. In Iranian traditional medicine, coriander has been indicated for a number of medical problems such as dyspeptic complaints, loss of appetite, convulsion and insomnia (13, 14).
In the current study, the effects of nickel nanoparticles on the growth factors of plant Corianderum sativum as well as the toxic activities of these nanoparticles have been investigated.
Materials and Methods
Nanoparticle
The nickel nanoparticles were procured from Nanoamor Co., Iran. Average particle size of experimental nanoparticle was 20 nm and purity was 99.5%. The shape of Ni nanoparticles was spherical. The nanoparticles were weighed and dispersed in distilled water at concentrations 20, 40 and 80 ppm. Then, they were sterilized at 120°C for 30 minutes. The nanoparticles were dispersed easily without precipitation by ultrasonicator and agglomerates were broken up with sufficient shaking. At the end, the nanoparticles were dispersed under suction for 20 minutes before use.
Planting and Harvesting
Corianderum sativum L. seeds were purchased from Pakan Bazr Co., Isfahan, Iran. The seeds were planted in 48 vases at green house of Zabol University of Medical Sciences. Every other day, the seeds were irrigated with 25 ml water for 3 months. Then, they were treated with 25 mL of different nickel nanoparticle concentrations namely, 20, 40 and 80 ppm every other day for 22 days (Fig 1). The plantlets were then collected and placed in separate aluminum foils (Fig. 1) and were stored at a -80°C freezer.
 |
Figure 1: Different stages of planting and harvesting of plant material. |
Measurement the Length of Root and the Aerial Part
Of the 6 coriander plants were randomly selected from each of the control and treated groups and their roots and stems length were measured by a ruler with an accuracy of 1 mm.
Relative Water Content (RWC) Measurement
In order to examine the relative water content of the plant aerial parts and roots, their fresh weight (FW), dry weight (DW) and turgid weight (TW) were determined. Immediately after harvesting, the plants were weighed to measure the fresh weight. The plants (0.5 g) were soaked in 100 ml of distilled water at 4°C in the dark for 24h in order to obtain turgid weight. Then, they were left for 48 h at room temperature until they were completely dry and were weighed to determine the dry weight. The relative water content (RCW) was determined by subtracting fresh weight from dry weight and multiplying this number by the difference between turgid weight and dry weight. RWC was calculated according to Smart and Bingham (15), using the following equation:
RWC = [fresh weight- dry weight/ turgid weight – dry weight] × 100
Photosynthetic Pigment Measurement
The photosynthetic pigment contents of the plants were measured according to the protocol described by Lichtenthaler and wellburn (16) in both control and treated samples. Three hundred mg of plant aerial parts (blank and treated samples) were powdered using liquid nitrogen. Then the volume was brought to 25 ml adding 80% acetone. The solution was centrifuged at 4800 rpm for 20 minutes. The absorbance of supernatant was measured to examine the contents of chlorophyll II a, b and carotenoids. For this purpose, the observance of the clear supernatant was read at 663.2, 663, 646.8, 645, and 470 nm (Shimadzo spectroscopy, A160 model, Japan). The following formula was used to calculate the pigment concentrations:
Chl a (µg/ml) = 12.25 × A663.2 – 2.79× A646.8
Chl b (µg/ml) = 21.5 × A646.8 – 5.1 × A663.2
Chl a +Chl b (µg/ml) = 7.15 × A663.2 + 18.71× A646.8
β-caroten (µg/ml) = (1000 ×A470 – 1.82 ×Ca – 85.02 × Cb)/198
Measurement of% Ni Nanoparticles in Total Ash
Two g of both treated and control plants were powdered and left for 4.5 h at 500-550 ºC in the oven. Then, the obtained ashes were weighted and mixed with 15 ml HCL 2M. The mixture was heated until the volume was 10 ml. After filtering, the obtained solution was mixed with water to get 50 ml. Finally, the absorbance of samples were measured with atomic absorption spectrometry.
Antioxidant Activity
To determine the antioxidant activity of treated and untreated plants, DPPH method was used. For this purpose, the concentrations 100, 50, 25, and 12.5µg/ml of methanol extract of the plants were prepared. Two ml of each sample was mixed with 1 ml methanol DPPH (0.2 µM) and were shaken. Then, all samples were incubated in the dark at room temperature for 30 min. the absorbance was measured at 517 nm using a UV-vis spectrophotometer. Inhibition percentage of DPPH is calculated as followed:
% Inhibition= (B0 – B1)/ B0 × 100
Where B0 and B1 represent absorbance of control and absorbance of the sample, respectively. In this assay, Vitamin E and butylated hydroxyanisole (BHA) were used as standards.
Statistical Analysis
The experimental design was randomized complete block and each reported value is the average of three replicates. Microsoft Excel 2007 program was used to calculate and represent raw data. The variances were analyzed by SPSS (version 11.5) software. Variance was used to analyze the quantitative changes of different parameters (ANOVA). All results are reported as mean ± standard deviation (SD). The results were considered significant if P<0.05, compared to control group.
Results
Nickel Nanoparticles Effects on Root and Shoot Elongation
To study the effects of nickel nanoparticle on plant length, six samples were selected randomly from control and treated groups (20, 40, and 80 ppm). As shown in figure 2, nickel nanoparticles decreased the growth of the root and shoot elongation when compared to the control group. Although all concentrations caused decrease in plant length, only the differences at concentrations 40 and 80 ppm were significant compared to the control group (**P<0.01). Comparing all samples with each other shows that the difference between concentrations 20 and 80 ppm is significant (*P<0.05).
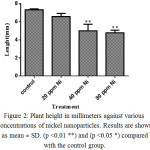 |
Figure 2: Plant height in millimeters against various concentrations of nickel nanoparticles. Results are shown as mean ± SD. (p <0.01 **) and (p <0.05 *) compared with the control group. |
Nickel Nanoparticles Effects on RWC
Relative water content (RWC) is an index demonstrating the amount of water in the plant organs and shows the ability of a plant in maintaining water under stress conditions. Therefore, in an experimental controlled environment, the measured RWC clearly showed the response of a plant. The higher the measured amount, the greater the ability of the treatment to preserve water (17). To determine the effect of nickel nanoparticles on the weight of plant parts, six plants from each control and treated groups were selected randomly and weighed.
Table 1: The mean dry weight in grams coriander plant against different concentrations of nickel nanoparticles.
| C. sativum | ||||
| Root | Shoot | |||
| FW (g) | DW (g) | FW (g) | DW (g) | |
| Control | 0.06 ± 0.01 | 0.02 ± 0.01 | 0.74 ± 0.01 | 0.15 ± 0.01 |
| 20 ppm Ni | 0.2 ± 0.1** | 0.04 ± 0.01** | 0.85 ± 0.01** | 0.24 ± 0.01** |
| 40 ppm Ni | 0.21 ± 0.01** | 0.05 ± 0.01** | 0.35 ± 0.01** | 0.09 ± 0.01** |
| 80 ppm Ni | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.18 ± 0.01** | 0.04 ± 0.01** |
Results are shown as mean ± SD. (p <0.01 **) compared with the control group
Nickel Nanoparticles Effects on Photosynthetic Pigments
Figure 3 shows the effects of nickel nanoparticles on photosynthetic pigment contents, including chlorophyll a, b, and carotenoids. The effect of nickel nanoparticles on photosynthetic pigment content is very different dependent on the type of pigment and nickel concentrations. Chlorophyll a was increased at concentrations 20 and 80 ppm while it decreased at concentration 40 ppm. In contrast, the content of chlorophyll b was decreased at concentrations 20 and 80 ppm whereas it decreased at 40 ppm concentration. The level of carotenoids was almost the same as control group; however it decreased at 40 ppm and increased at 80 ppm.
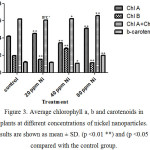 |
Figure 3. Average chlorophyll a, b and carotenoids in plants at different concentrations of nickel nanoparticles. Results are shown as mean ± SD. (p <0.01 **) and (p <0.05 *) compared with the control group. |
Nickel Nanoparticles Effects on Total Ash
The effect of nickel nanoparticles on % total ash of coriander plant is shown in figure 4. The content of %total ash was significantly different in the plants treated with nickel nanoparticles at concentrations 20 ppm (*P < 0.05), and 40 and 80 ppm (*P < 0.001).
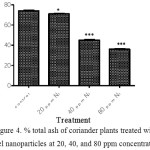 |
Figure 4: % total ash of coriander plants treated with nickel nanoparticles at 20, 40, and 80 ppm concentrations. |
Nickel Nanoparticles Content in Plants
The amount of nanoparticles in each treatment of the plant was determined by atomic absorption spectrometry and the standard curve was depicted (Figure 5). According to the line equation obtained, the concentration of nanoparticles in each treatment was calculated. The results are shown in Table 2.
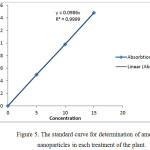 |
Figure 5: The standard curve for determination of amount of nanoparticles in each treatment of the plant. |
Table 2: The concentration of nickel nanoparticles in the plants treated by 20, 40, and 80 ppm concentrations (n=3).
| Treatment | Concentration |
| 20 ppm Ni | 0.02 |
| 40 ppm Ni | 0.036 |
| 80 ppm Ni | 0.045 |
Nickel Nanoparticles Effects on Antioxidant Activity
To determine the antioxidant activity of coriander plants treated by various concentrations of nickel nanoparticles, the IC50 values were calculated (Table 3). Vitamin E was used as a standard natural antioxidant and BHA as a synthetic one. The results showed that as the concentration of nanoparticles increased; the IC50 was increased as well, showing that the antioxidant activity of the plant was decreased.
Table 3. The IC50 values of coriander plants treated with and without nickel nanoparticles at concentrations 20, 40, and 80 ppm, as well as Vit E and BHA.
| Concentration (µg/mL) | Control | 20 ppm Ni | 40 ppm Ni | 80 ppm Ni | BHA | Vit E |
| IC50 | 30±10 | 31±10 | 33±10 | 34±10 | 16±10 | 15±10 |
Discussion
The results of the present study showed that as the concentration of nickel nanoparticles increases, the growth of the plant decreases. In 2007, Lin et al. studied the phytotoxicity of nanoparticles and their inhibitory activity on germination and growth of seeds and roots (18). They examined the toxicity of Al2O3 nanoparticles at concentrations 20, 200, and 2000 mg/l in plants radishes, corn, lettuce, cucumbers, and canola. They found this nanoparticle phytotoxic only at the highest concentration. In addition, the phytotoxicity of Zn and ZnO was significant and decreased the root elongation of the crns tested. They found that the higher the concentration of the nanoparticle, the lesser the root elongation. The toxicity of nickel nanoparticles has been also investigated by different research groups. For instance, Gong et al. have studied the biotoxicity of nickel oxide nanoparticles (19). They determined the toxicity of NiO nanoparticles at concentrations 10-50 mg/L on Chelorella vulgaris. They realized that this nanoparticle significantly inhibits the growth of the plant in a concentration dependent manner (P < 0.01). They proposed nanoparticle aggregation as a possible mechanism of the nanoparticle phytotoxicity.
Taken together, it can be concluded that nanoparticles are phytotoxic at high concentrations. The absence of toxicity at lower concentrations is duo to homeostasis in plant cells. This phenomenon gives the plant the ability to grow under this situation. The future studies should focus on molecular mechanisms of nanoparticles toxicity to reveal the mechanisms of actions.
The results showed that nickel nanoparticles decreased the fresh and dry weight of aerial parts significantly (P < 0.01) and they did not have a significant effect on the roots. In 2012, Sheng-Jiang et al. studied the toxicity of AgNO3 and silver nanoparticles on Spiradelha polyrhiza (20). The fresh and dry weight of the plants decreased in a concentration dependent of the nanoparticles. At the highest concentration of silver nanoparticles (10 mg/l), the weights decreased 50% compared with the control group. The phytotoxicity of nanoparticles has been investigated in several other studies. For example, the effects of silver nanoparticles (5, 10, 20, 40, 80, and 160 µg/L) were studied on Lemna minor (21). They also revealed the toxicity of silver nanoparticles on weights of plants. The results of our study have confirmed the results of previous studies. Nanoparticles, at high concentrations, decrease the fresh and dry weight of both roots and aerial parts. Further studies are required to uncover the precise mechanisms of action.
Nickel nanoparticles increased the levels of photosynthetic pigments including chlorophyll a, b, and carotenoids at high concentrations. this trend has also been observed by other research groups (22). There are several proposed mechanisms for this increase in photosynthetic pigments. In general, it is suggested that different nanoparticles cause damages in the photosynthetic process. Thus, plants increase the production of these pigments to make a balance and cellular homeostasis. Type of nanoparticle, plant species, size and shape of nanoparticles are other factors influencing this phenomenon. It is recommended that in order to clarify the involved mechanisms, each of the mentioned factors to be considered separately.
The results showed a significant decrease in the amount of total ashes dependent on the nanoparticle concentrations (P < 0.001). According to literature survey we did, there is no similar study in this regard. However, duo to the phytotoxicity effects we observed in other morphological and biochemical factors, this weight loss in total ash can be attributed to decrease in mineral constituents of plants.
The nanoparticles levels increased in the plants as the concentration of them increased in the environment of the plants. This result suggests the environmental pollution with nanoparticles is easily transferable to the plants and as a consequence to the food chain of humans. On the other hand, this result shows that the morphological and biochemical changes observed are correlated to the increase in the nanoparticles.
The antioxidant activity of the plants decreased in a concentration dependent of nickel nanoparticles. According to our knowledge, there is no previous study for investigating the effects of nickel nanoparticles on antioxidant activities. However, the inhibition of radical scavenging activity of the plant metabolites is a possible mechanism.
In conclusion, the nickel nanoparticles showed phytotoxic effects on morphological and biochemical properties of Coriandrm sativum. However, the precise mechanisms underlying should be investigated in future studies.
Acknowledgements
Conflict of interest
The authors declare no conflict of interest.
References
- Rad MS, Rad JS, Heshmati GA, Miri A, Sen DJ. Biological synthesis of gold and silver nanoparticles by Nitraria schoberi fruits. American Journal of Advanced Drug Delivery. 2013;1(2):174-9.
- Rad JS, Alfatemi MH, Rad MS, Rad MS, Sen DJ, Mohsenzadeh S. In-vivo titanium dioxide (TiO2) nanoparticles effects on chromosomal abnormalities and lactate dehydrogenase activity. American Journal of Advanced Drug Delivery. 2013;1(3):232-7.
- Sharifi-Rad J, Hoseini‑Alfatemi S, Sharifi-Rad M, Iriti M. Antimicrobial synergic effect of Allicin and silver nanoparticles on skin infection caused by methicillin resistant Staphylococcus aureus spp. Annals of medical and health sciences research. 2014;4(6):863-8.
CrossRef - Sharifi Rad J, Karimi J, Mohsenzadeh S, Rad MS, Moradgholi J. Evaluating SiO2 Nanoparticles Effects on Developmental Characteristic and Photosynthetic Pigment Contents of Zea mays L. Bulletin of Environment, Pharmacology and Life Sciences. 2014;3(6):194-201.
- Daniel MC, Astruc D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications Toward Biology, Catalysis, and Nanotechnology. Chem Rev. 2004;104(1):293-346.
CrossRef - Shi D, Sadat ME, Dunn AW, Mast DB. Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications. Nanoscale. 2015;7(18):8209-32.
CrossRef - Venkatesan K, Rajan Babu D, Kavya Bai MP, Supriya R, Vidya R, Madeswaran S, et al. Structural and magnetic properties of cobaltdoped iron oxide nanoparticles prepared by solution combustion method for biomedical applications. Int J Nanomed. 2015;10:189-98.
- Walkey C, Das S, Seal S, Erlichman J, Heckman K, Ghibelli L, et al. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ Sci Nano. 2015;2(1):33-53.
CrossRef - Ball P. Natural strategies for the molecular engineer. Nanotechnology. 2002;13(5):R15-R28.
CrossRef - Siddiqui MH, Al-Whaibi MH, Mohammad F. Nanotechnology and plant sciences: Nanoparticles and their impact on plants: Springer International Publishing; 2015. 1-303
CrossRef - Thul ST, Sarangi BK. Implications of nanotechnology on plant productivity and its rhizospheric environment. Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants: Springer International Publishing; 2015. 37-53.
- Wang C-B, Gau G-Y, Gau S-J, Tang C-W, Bi J-L. Preparation and characterization of nanosized nickel oxide. Catal Lett. 2005;101(3-4):241-7.
CrossRef - Mahendra P, Bisht S. Coriandrum sativum: A daily use spice with great medicinal effect. Pharmacogn J. 2011;3(21):84-8.
CrossRef - Chawla S, Thaku M. Coriandrum sativum: A promising functional and medicinal food. Med Plants. 2013;5(2):59-65.
- Haghighi M, Pourkhaloee A. Nanoparticles in agricultural soils: Their risks and benefits for seed germination. Minerva Biotecnol. 2013;25(2):123-32.
- Lichtenthaler HK, Wellburn AR. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochemical Society Transactions. 1983;11(5):591-2.
CrossRef - Abbaszadeh B, Ashourabadi ES, Lebaschi MH, Kandy MNH, Moghadami F. The Effect of Drought Stress on Proline Contents, Soluble Sugars, Chlorophyll and Relative Water Contents of Balm. Iranian Journal Of Medicinal And Aromatic Plants. 2008;4:504-13.
- Lin D, Xing B. Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut. 2007;150(2):243-50.
CrossRef - Gong N, Shao K, Feng W, Lin Z, Liang C, Sun Y. Biotoxicity of nickel oxide nanoparticles and bio-remediation by microalgae Chlorella vulgaris. Chemosphere. 2011;83(4):510-6.
CrossRef - Jiang HS, Li M, Chang FY, Li W, Yin LY. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ Toxicol Chem. 2012;31(8):1880-6.
CrossRef - Gubbins EJ, Batty LC, Lead JR. Phytotoxicity of silver nanoparticles to Lemna minor L. Environ Pollut. 2011;159(6):1551-9.
CrossRef - Sharifi Rad J, Mohsenzadeh S, Sharifi Rad M, Moradgholi J. Evaluating SiO2 Nanoparticles Effects on Developmental Characteristic and Photosynthetic Pigment Contents of Zea mays L. Academy for Environment and Life Sciences. 2014;3(6):194-201.

This work is licensed under a Creative Commons Attribution 4.0 International License.









