Green Protocols for the One-Pot Synthesis of Vanillin Based Aminoalkyl and Amidoalkyl Naphthols and their Antibacterial Activity
Afinisha Deepam and Jyothi Viswanadhan
Department of chemistry, Christian college, Kattakada, Thiruvananthapuram, Kerala.
Corresponding Author E-mail: afinishadpm@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330336
Environment-friendly, facile, cost effective green chemical methods are reported for the one-pot multicomponent solvent less synthesis of 1-aminoalkyl-2-naphthol and 1-amidoalkyl-2-naphthol exploring tannic acid as a novel, biodegradable, cheap and efficient Lewis acid catalyst. Present investigation focuses on green chemistry approach for the synthesis of aminoalkyl and amidoalkyl compounds. A mixture of sufficient amount of tannic acid, vanillin, 2-naphthol and 4-nitroaniline/ benzamide is refluxed by following various green protocols such as oil bath, microwave irradiation, hot plate with magnetic stirrer, Grindstone method and finally conventional heating method. Structure of the products confirmed by spectroscopic techniques such as IR, 1H NMR, 13C NMR and GC-MS. Anti-bacterial activity of synthesized compounds against Bacillus subtilis is tested by zone inhibition method.
KEYWORDS:Amidoalkyl naphthol; Aminoalkyl naphthol; microwave synthesis; Grindstone chemistry; Oil bath; Vanillin; 2-naphthol
Download this article as:| Copy the following to cite this article: Deepam A, Viswanadhan J. Green Protocols for the One-Pot Synthesis of Vanillin Based Aminoalkyl and Amidoalkyl Naphthols and their Antibacterial Activity. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Deepam A, Viswanadhan J. Green Protocols for the One-Pot Synthesis of Vanillin Based Aminoalkyl and Amidoalkyl Naphthols and their Antibacterial Activity. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=32981 |
Introduction
Green chemistry is defined as sustainable chemistry, which is dealing with environment- friendly chemical synthesis. It is the design of chemical products and processes that reduce or eliminate the use of hazardous substances. Green chemistry covers the entire life cycle of a chemical product and aims to reduce hazardous substances from the design process, manufacturing, use, and final disposal. Solvents using in industry and volatile organic compounds (VOCs) lead to environmental pollution and risks to human health. One of the important green path way to reduce pollution is solvent less synthesis; mainly in the field of organic chemistry. The toxicity and volatile nature of many organic solvents, particularly chlorinated hydrocarbons that are widely used in huge amounts for organic reactions have posed a serious threat to the environment. Thus, design of solvent less catalytic reaction has received tremendous attention in recent times. The drive for the development of dry media reactions in chemistry is the method is economics (save money on solvent), to avoid removal of a solvent after reaction completion, reaction rate is high due to more availability of reactants, environmentally friendly because solvent is not required and simple to handle. Solvent-less reactions can be successfully performed in the case of multicomponent reactions. One pot multicomponent reactions (MCR), sometimes called as zipper reactions have gained wide attention in the field of synthetic organic chemistry as they increase the efficiency of the reaction and decrease the number of laboratory operations along with quantities of solvent and chemicals used. They also reduce the reaction time considerably and facilitate the yield of products than the normal multiple step methods. MCR link several transformations in a single step. MCR is an important tool in reactions such as Biginelli, Ugi, Mannich and Betti reactions. The Betti reaction is an addition reaction between aldehydes, primary aromatic amines and phenols producing α-aminobenzylphenols. The product of the Betti reaction is called the Betti base. Betti bases are said to be aminoarylphenols.
1-amidoalkyl-2-naphthols are important compounds in the field of synthetic organic chemistry because it is building block for the synthesis of many vital compounds. There are remarkable intermediates which can be easily converted into biologically active 1-aminoalkyl-2- naphthol derivatives by amide hydrolysis. The hypotensive and bradycardiac effects of these compounds have been evaluated1. Also, amidoalkyl naphthols can be used for the synthesis of highly functionalized oxazine derivatives through Vilsmeier cyclisation reaction2. In Vilsmeier cyclisation reaction, the oxazine derivatives are prepared from corresponding amidoalkyl naphthols by treating it with Vilsmeier reagent at room temperature. The reagent is a mixture of 12 equivalence of DMF (Dimethylformamide) and 8 equivalence POCl3. This experiment is based on the mechanism of Vilsmeier- Haack reaction which involves the synthesis of functionalized heterocycles. Molecules having 1, 3- oxazine moiety is important due to their large spectrum of pharmacological applications such as anti-tumor3, anti-malarial and anti-microbial activities4. Because of its wide range of applications, synthesis of amidoalkyl naphthol derivatives has attracted the attention of chemists and various improved procedures have been reported.
Bronsted or Lewis acid catalysts have been utilized for the preparation of 1- amidoalkyl- 2-naphthols. This type of compounds can be prepared by the condensation of aromatic aldehydes, 2-naphthol and amides, urea or acetonitrile in the presence of a Lewis or Bronsted acid catalysts. Several methods have been documented in the literature for synthesis of these compounds using a variety of catalysts such as Montmorillonite K10 clay5 Ce (SO4)26, benzimidazolium based ionic liquid7, Butyl methyl imidazolium bromide8, N,N,N’,N’-Tetrabromobenzene- 1,3-disulfonamide (TBBDA)9, Carbon based solid acid10, Pentafluorophenylammonium Triflate (PFPAT)11, Molten tetraethyl ammonium chloride12, Boric acid 13, polyphosphate ester 14 etc. But majority of these reactions need high temperature, long time, expensive reagents, high catalyst loading, corrosive reagents, strongly acidic conditions and low yields of products. Therefore introduction of a suitable ecofriendly catalyst and efficient methods for the preparation of 1-amidoalkyl-2-naphthols and 1-aminoalkyl-2-naphthols are of practical importance.
Tannic acid, a naturally occurring plant polyphenol is used as the mild Lewis acid catalyst for the synthesis of aminoalkylnaphthols, amidoalkylnaphthols 15 and some oxazine derivatives. The chemical formula for commercial tannic acid is given as C76H52O46, i.e., a molecule with deca-galloyl glucose. (Gallic acid ester of glucose).
But sometimes exists as a mixture of polygalloyl glucoses or polygalloyl quinic acid esters with the number of galloyl moieties per molecule ranging from 2 up to 12. It is depending on the plant source used to extract the tannic acid. Tannic acid contains central glucose molecule derivatised at its hydroxyl groups, with one or more galloyl residues. Its weak acidity (pKa around 10) is due to the numerous phenol groups in the structure. Tannic acid exists in two forms. Quercitannic acid found in oak bark and leaves and Gallotannic acid found in oak galls. Molar mass of tannic acid is 1701.19g/mol and is highly soluble in water (2850g/L). Commercial tannic acid is usually extracted from plant parts such as Caesalpinia spinosa (commonly known as Tara pods or Quechua is a small leguminous tree or thorny shrub native to Peru), gallnuts from Rhus semialata (Chinese sumac or nutgall tree) or Quercus infectoria (a species of oak, bearing galls that have been traditionally used in Asia as medicine).
Strong acids cannot be used for catalyzing multicomponent organic synthesis which involve aldehydes. Because use of acidic catalysts in aldehydes bearing basic groups or acid sensitive aldehydes is not possible. Polyphenolic nature of tannic acid may be explored for their catalytic efficiency. Tannic acid is successfully used as mild Lewis acid catalyst in the Betti type multicomponent synthesis of amidoalkyl naphthols 15 and 2, 4, 5-triaryl-iH-imidazole 16.
Solvent less synthesis of amidoalkyl and aminoalkyl naphthols have been done by adopting various methods such as microwave irradiation, heating on an oil bath, heating on hot plate with magnetic stirring and Grindstone chemistry method 17. As already mentioned, various pharmaceutical applications of amidoalkyl naphthols as well as aminoalkyl naphthols are reported. Vikas Verma et al reported the synthesis of 1, 3- disubstituted-1H-naphthol [1, 2-e] [1, 3] oxazines and evaluation of in vitro antibacterial activity against Gram-positive Bacillus subtillis [MTCC 2063], Staphylococcus aureus [MTCC 2901] and Gram-negative E18. coli [MTCC 8184]. These compounds are synthesized from amidoalkyl naphthols. Also, antibacterial activities of some amidoalkyl naphthols (synthesized using nano- TiSiO2 as catalyst) against standard species of S. aureus [MTCC 25293], En. Faecalis [MTCC 11700] and P. aeruginosa [MTCC 27853] are discovered by 19. Synthesized compounds are tested for their anti-bacterial activity using zone inhibition method. Zone Inhibition Test, also called a Kirby-Bauer Test, is a qualitative method used clinically to measure antibiotic resistance and industrially to test the ability of solids and textiles to inhibit microbial growth. Researchers who develop antimicrobial textiles, surfaces, and liquids use this test as a quick and easy way to measure and compare levels of inhibitory activity.
In this work, we synthesized and characterized one compound from each type of the compounds aminoalkyl and amidoalkyl naphthols through different methods such as oil bath, microwave irradiation, hot plate with magnetic stirrer and Grindstone chemistry method. The reactants used are aromatic aldehyde (vanillin), 2- naphthol and aromatic amine (4-nitro aniline) or aromatic amide (benzamide) for getting aminoalkyl and amidoalkyl naphthols respectively. Also, the two compounds are tested for their anti-bacterial activity against Bacillus Subtilis. It is known also as the hay bacillus or grass bacillus, a Gram-positive, catalase-positive bacterium, found in soil and the gastrointestinal tract of ruminants and humans.
Materials and Methods
All chemicals and solvents were purchased from central scientific laboratory supplies, Panavila, Thiruvananthapuram. The melting points were determined by an open capillary method and are uncorrected. All chemicals used are of analar grade. Reactants used are 2-naphthol, vanillin, p-nitroaniline, benzamide and tannic acid. Solvents used are ethanol, chloroform, ethyl acetate, hexane, acetone, DMSO etc.
Synthesis of two compounds were done by following different methods using the naturally occurring plant polyphenol tannic acid. Compound A is an aminoalkyl naphthol which is synthesized from 2-naphthol, vanillin and 4-nitroanilin and compound B is an amidoalkyl naphthol synthesized by the condensation of 2- naphthol, vanillin and benzamide.
Procedure for the synthesis of Aminoalkyl and Amidoalkyl Naphthols via Different Methods.
Method I- Oil Bath
A mixture of 2-naphthol, vanillin and p-nitroaniline is taken in a ratio 1:1:1.3 mmol (0.144: 0.152: 0.1795) and 0.02 mmol of tannic acid (0.03402g) mixed in a 100ml conical flask. In another conical flask, mixture of 2-naphthol, vanillin and benzamide (0.144: 0.152: 0.1574 g) and 0.03 mmol of tannic acid (0.05103g) is taken. Both the reaction mixtures are heated in oil baths of cotton seed oil kept in hot plate at 120-125oC for 10-15 min. To know whether the reaction is complete, samples are taken from the reaction mixture at constant intervals of time and TLC is performed in each case. After the completion of reaction, spot for the product is detected by performing TLC using the same solvent system (hexane: ethyl acetate-1:4) and Rf values are compared with those obtained for reactants. After completing the reaction, the solid form of crude product is cooled to room temperature, washed with water to remove water soluble unreacted vanillin and tannic acid. Finally, pure product is obtained through the recrystallization using ethanol (EtOH: H2O – 1:3) 20.
Method II- Microwave Irradiation
Reaction mixture is 2- naphthol, vanillin, and p-nitroaniline (or benzamide) in the ratio 1:1:1.3 mmol added with 0.02 mmol (or 0.03 mmol) of tannic acid and it was then placed in a microwave oven (Model no: MW73AD-B/XTL, SAMSUNG 800W), at 100W. Each pulse of 30 s with intermittent time is applied to avoid overheating. The time taken for completing the reaction is determined by performing the reaction for 1-4 min. The completion of reaction checked by TLC. After completion of reaction, the mixture is cooled to room temperature and washed with cold water. Pure crystals recrystallized from the crude product using ethanol.
Method III- Hot Plate With Magnetic Stirrer
A mixture of vanillin, 2-naphthol, and p-nitroaniline (or benzamide) in 1:1:1.3mmol is taken in a white marble mortar and pestle. It is grounded well with o.o2mmol (0.03mmol) of tannic acid and transferred into a 100ml conical flask and stirred after keeping magnetic fish. The mixture is then heated in hot plate (REMI 2MLH) at about 120-125oC. The process is continued for 20-25 min keeping the temperature constant and regulating the speed of stirring. The completion of the reaction is checked by TLC using the solvent system hexane: ethyl acetate (4:1). The resulting mass was cooled, extracted with water, and recrystallized from ethanol.
Method IV- Grindstone Chemistry Method
A mixture of reactants (2-naphthol, vanillin, and p-nitroaniline (or benzamide) in the ratio 1:1:1.3 mmol is taken in marble mortar and finely ground using pestle for 10-15 min with catalyst, tannic acid powder (0.02 or 0.03mmol). The reaction is checked by TLC, then the resultant mass was washed with water and the crystals of final product are obtained by recrystallization using ethanol.
Method V- Normal Refluxing
A mixture of reactants and catalyst in the same ratio as described above is refluxed with a few drops of methanol for 60-90 min. The reaction progress was followed by TLC. Pure product is separated by washing with water and recrystallization using ethanol.
Purification of products is done by column chromatography. The column is packed with silica gel (200-400). The crude product is dissolved in chloroform and loaded in the column. It is eluted with hexane: ethyl acetate of ratio 1:1 8. The eluted compounds are separated by TLC using solvent system Hexane: ethyl acetate (4:1) and developed spots were visualized after keeping in iodine chamber and Rf value is noted.
Ultraviolet-Visible spectra of purified products are obtained by dissolving in ethanol and recorded in UV-Visible spectrometer (UV-Vis-NIR Spectrophotometer, Varian Cary 5000 with wavelength range 175-3300nm). IR spectra is recorded in IR spectrometer (Schimadzu IR prestige-20 spectrometer) with KBr as the reference compound and compared the values with that of reactants. The products were also analyzed using 1H NMR, 13C NMR (NMR spectrometer, Bruker Avance III, 400MHz) and GC-MS in Thermo Exactive Orbitrap (Gas Chromatography Mass spectrometer, Shimadzu 5050A-Model) techniques.
The two products A and B are subjected to zone inhibition test to study about their anti- bacterial activity. A culture of bacterium Bacillus subtilis is allowed to grow in a petri plate in agar medium. Using a sterile swab, a suspension of the pure culture is spread evenly over the face of a sterile agar plate. It is stored 24-48 h for attaining normal bacterial growth. The compounds are introduced into the holes bored in the plate and incubated overnight. Size of the zone visible around the hole is measured.
Results and Discussion
To study the synthesis of amidoalkyl and aminoalkyl naphthols, Betti reaction is considered as a standard reaction. Betti reaction is the condensation reaction of aromatic aldehydes, primary aromatic or heterocyclic amines and phenols leading to α-aminobenzylphenols.
Compound A: Aminoalkyl naphthol, which is prepared by the condensation of 2-naphthol, vanillin and 4-nitroaniline in presence of tannic acid as catalyst.
Compound B: Amidoalkyl naphthol, which is prepared by the condensation of 2-naphthol, vanillin, and benzamide in presence of tannic acid as catalyst.
Monitoring reaction progress and detection of formed products were done using TLC and Rf values are calculated. This data can be taken as the primary awareness about our products. The Rf values of reactants are determined by conducting TLC of each of them in the solvent system, hexane: ethyl acetate (4:1). Rf values are shown in Table 1.
Table 1: RF Values of Reactants in a Solvent System of Hexane: Ethyl acetate (4:1).
| No | Name of reactant | Rf value |
| 1 | 2-naphthol | 0.8064 |
| 2 | Vanillin | 0.2129 |
| 3 | 4-nitroaniline | 0.3870 |
| 4 | Benzamide | 0.0903 |
TLC of purified products and reactants were conducted in the above solvent system and compared Rf values with that of reactants and new bands are identified viz: Product A (Rf = 0.4) and product B (Rf = 0.74). The optimization study of reactants is determined by keeping amount of 2-naphthol and vanillin as constant and varying third constituent (4-nitroaniline or benzamide). The reaction is done by refluxing in cotton seed oil bath. Results are tabulated in Table 2.
Table 2: Determination of Ratio of Reactants to get Maximum Yields to get Products a and b.
| Amount of reactants (mmol) | Yield of product A (%) | ||
| Product A- aminoalkyl naphthol | |||
| Vanillin | 2-naphthol | p-nitroaniline | |
| 1 | 1 | 1 | 64 |
| 1 | 1 | 1.1 | 72.32 |
| 1 | 1 | 1.2 | 81.9 |
| 1 | 1 | 1.3 | 90.01 |
| 1 | 1 | 1.4 | 84.92 |
| 1 | 1 | 1.5 | 82.608 |
| Product B- amidoalkyl naphthol | Yield of product B (%) | ||
| Vanillin | 2-naphthol | Benzamide | |
| 1 | 1 | 1 | 76.9 |
| 1 | 1 | 1.1 | 84.3 |
| 1 | 1 | 1.2 | 87 |
| 1 | 1 | 1.3 | 92.1 |
| 1 | 1 | 1.4 | 89.76 |
| 1 | 1 | 1.5 | 86.85 |
From the table, it is clear that 2-naphthol: vanillin: 4-nitroaniline / benzamide in the ratio 1:1:1.3 mmol is yielded a higher percentage of products both A and B. The present ratio is in agreement with many reports. 2-naphthol: Aromatic aldehyde: Aromatic amine/amide in the ratio 1:1:1.3 mmol 21and 1:1:1.2 mmol 7, 15 were found to yield higher percentage. Variation on the yield of A and B is plotted against concentration of 4-nitroaniline/ benzamide shown (seeFig. 1 and 2).
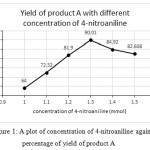 |
Figure 1: A plot of concentration of 4-nitroaniline against percentage of yield of product A Click here to View figure |
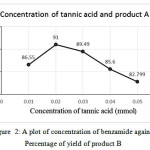 |
Figure 2: A plot of concentration of benzamide against Percentage of yield of product B Click here to View figure |
The optimization study of tannic acid is also done by repeating the experiment with different amount of tannic acid were the reactants taken in the ratio 1:1:1.3 mmol. Table 3 shows the yield of compounds A and B when different concentrations of tannic acid were used. Graphs were plotted with amount of catalyst against yield of products and compared the results (SeeFig 3 and 4) shows the variation in the yield of products with different concentrations of catalyst, tannic acid.
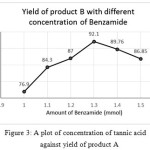 |
Figure 3: A plot of concentration of tannic acid against yield of product A
|
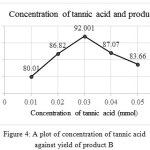 |
Figure 4: A plot of concentration of tannic acid against yield of product B
|
Table 3: Concentration of Tannic acid and the Yield of Products
| Concentration of catalyst, tannic acid (mmol) | Yield of products (%) | |
| Compound A | Compound B | |
| 0.01 | 86.55 | 80.01 |
| 0.02 | 91.0 | 86.82 |
| 0.03 | 89.49 | 92.001 |
| 0.04 | 85.60 | 87.07 |
| 0.05 | 82.799 | 83.66 |
From this, 0.02 mmol of tannic acid yielded maximum of product A and 0.03 mmol of tannic acid gave maximum amount of product B when the ratio of reactants taken in both reactions as 1:1:1.3 mmol (Table 4). The result is agreeing with the synthesis of derivatives of amidoalkyl naphthols using tannic acid catalyst15. 0.02 mmol and 0.03 mmol of tannic acid in reactions to get products A and B respectively gave maximum amounts of products and no more addition or removal of catalyst can cause increase in the yield of products.
Table 4: Yield of Products a and b from Various Methods of Synthesis
|
No |
Method of synthesis | Yield (%) | |
| Product A(Aminoalkyl naphthol) |
Product B (Amidoalkyl naphthol) |
||
| I | Oil bath | 90.01 | 92.1 |
| II | Microwave irradiation | 87.32 | 89.99 |
| III | Hot plate with magnetic stirrer | 82.045 | 83.3 |
| IV | Grindstone method | 77.921 | 36.32 |
| V | Normal refluxing | 52.76 | 54.367 |
Hence the reactants 2-naphthol: vanillin: p-nitroaniline (A) /benzamide (B) in the ratio 1:1:1.3 mmol with 0.02 mmol tannic acid to product A and 0.03 mmol of tannic acid to product B is used throughout the synthesis of compounds in different methods. The results obtained from above methods and yield of final products A and B are tabulated. Table 4 shows the yield of products A and B by following various methods.
Hence synthesis in oil bath is the best method to get higher percentage of product A and B. This method gave product A with 90.01% and product B with 92.10% of yield. Many experiments shown similar results in the synthesis of different derivatives belong to this category 7. Also, the result agreed with the report of 10, 22 etc.
Microwave irradiation of synthesis of products is in competitive with yield obtained by oil bath. The present catalyst, naturally occurring tannic acid is good enough to synthesize aminoalkyl as well as amidoalkyl naphthol (A and B) with 87-89% yields. This is comparable with the works done by Zare et al8 who prepared amidoalkyl derivatives in 85-90% yield with a synthetic ionic liquid 1-butyl-3-methylimidazolium bromide [Bmim] Br). The conditions they used were 800W and 130°C. In the present reaction conditions optimized is 480W and 120oC to get higher percentage of products.
Table 5: Determination of turn over frequency of tannic acid in different methods of synthesis
|
No |
Method of synthesis | Concentration of tannic acid (mol %) | Reaction time (min) | Yield of product (%) | TOF(min-1) | ||||
| A | B | A | B | A | B | A |
B |
||
|
I |
Oil bath | 2 | 3 | 12 | 14 | 90.01 | 92.1 | 3.750 | 2.193 |
| II | Microwave irradiation | 2 | 3 | 3.5 | 4 | 87.32 | 89.99 | 12.855 | 7.499 |
| III | Hot plate with magnetic stirrer | 2 | 3 | 23.5 | 24 | 82.045 | 83.3 | 1.7456 | 1.1563 |
| IV | Grindstone method | 2 | 3 | 10 | 14 | 77.921 | 36.32 | 3.896 | 0.8647 |
| V | Normal refluxing | 2 | 3 | 70 | 85 | 52.76 | 53.36 | 0.3768 | 0.3138 |
Synthesis using direct hot plate with magnetic stirrer is moderately efficient in the synthesis of products A and B when compared to microwave and oil bath heating. This trend is also reported by Rajesh et al 15. They explained that, low yield of products is due to non-uniform heating which may lead to the formation of undesired products. Grindstone chemistry method is applicable for the synthesis of compound A (77. 92%) but 17 reported that the synthesis of aminoalkyl naphthol derivatives with 95% of yield can be obtained by using the same method. Our experiments cannot achieve much higher yields of compound A by following the same method. In case of compound B, only 36.32% of product is separated. That shows the method
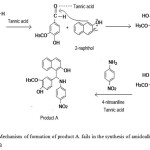 |
Scheme 1: Mechanism of formation of product A. fails in the synthesis of amidoalkyl naphthol, compound B Click here to View scheme |
To show the advantages, efficiency and applicability of tannic acid, turn over frequency (TOF) of the catalyst is calculated in different methods and compared with already reported works. Table 6 shows the TOF of tannic acid in various methods. Values of turn over frequency is tabulated in Table 5. Higher the TOF value, greater will be the efficiency of the catalyst. TOF calculations shows that, tannic acid is more efficient in the synthesis of products by green paths such as synthesis using oil bath and using microwave irradiation. TOF of tannic acid is higher for the synthesis of Product A (12.855 min-1).
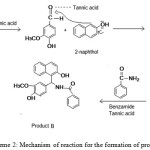 |
Scheme 2: Mechanism of reaction for the formation of product B Click here to View scheme |
Mechanism of Reactions and Structure of Products
Aldehyde group of vanillin is attacked by the Lewis acid, tannic acid to facilitate the addition of vanillin to α position adjacent to the carbon which contains -OH group. Then the intermediate complex eliminates -OH and a -H as a water molecule to form a double bond between 2-naphthol and -CH group of vanillin. This intermediate formed by the nucleophilic addition of 2-naphthol with vanillin belongs to a type of compound known as ortho-quinone methides 7, 13. When 4-nitroaniline added to this intermediate in presence of tannic acid, Michael addition occurs and product A is obtained. This mechanism is assigned with the help of many experiments on the synthesis of similar type of compounds which are already reported 7, 15, 17. IUPAC name and structure of product A is given (seeFig 5).
Aldehyde group of vanillin is attacked by the Lewis acid, tannic acid to facilitate the addition of vanillin to α position adjacent to the carbon which contains -OH group. Then the intermediate complex eliminates -OH and a -H as a water molecule to form a double bond between 2-naphthol and -CH group of vanillin. This intermediate formed by the nucleophilic addition of 2-naphthol with vanillin belongs to a type of compound known as ortho-quinone methides. When benzamide added to this intermediate in presence of tannic acid, Michael addition occurs and product B is obtained. This mechanism is assigned with the help of many published reports on the synthesis of similar type of compounds 7,13,15. IUPAC name and structure of product B is given (seeFig 5).
![Figure 5: Structure and IUPAC name of product A. 1-[(4-hydroxy-3-methoxyphenyl) (4-nitrophenylamino)] methyl naphthalene-2-ol](http://www.orientjchem.org/wp-content/uploads/2017/05/Vol33No3_Gree_AFIN_fig5-150x150.jpg) |
Figure 5: Structure and IUPAC name of product A. 1-[(4-hydroxy-3-methoxyphenyl) (4-nitrophenylamino)] methyl naphthalene-2-ol |
Anti-bacterial activity of products
Products A and B is tested for antibacterial activity against Bacillus subtilis. Compounds are introduced in concentrations 50 ϻl and 100 ϻl. It is found that both compounds shows negligible inhibition against the growth of this bacterium. Inhibition zone is measured only 1 mm range in diameter and it cannot be considered as an effective activity. Some modifications structure of compounds or in type of bacteria, storage conditions, growth of bacterium, size of plate etc may lead to better results. The petri plate showing this experiment is shown (seeFig 6).
![Figure 6: Structure and IUPAC name of product B. 1-[(4-hydroxy-3-methoxyphenyl) (phenylamido)] methyl naphthalene-2-ol](http://www.orientjchem.org/wp-content/uploads/2017/05/Vol33No3_Gree_AFIN_fig6-150x150.jpg) |
Figure 6: Structure and IUPAC name of product B. 1-[(4-hydroxy-3-methoxyphenyl) (phenylamido)] methyl naphthalene-2-ol
|
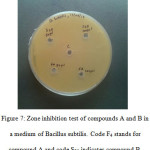 |
Figure 7: Zone inhibition test of compounds A and B in a medium of Bacillus subtilis. Code F4 stands for compound A and code S25 indicates compound B. |
Characterization Data
Product A
UV-VIS: λmax = 1232 nm (44.327%)
IR (KBr) (υmax/cm-1): 3062.96, 3022.45, 2974, 2947, 2848 (Ar-C-H and Ar-OH str), 817.8,761,729 (out of plane Ar-C-H bending), 1508-1458 (Ar-C=C str), 3404 (-N-H str), 1390-1377 (Ar-C-NO2 str), 1591 (out of plane -N-H bending), 3000-3500 (broad, hydrogen bonded -O-H ), 1508-1380 (deformation of -OH), 1271 (C-O-C str), 1377 (-C-O).
1H NMR (CDCl3, 400 MHz) (δ/ppm): 3.861 (s, 3H), 6.042 (s, 1H), 6.953-6.974 (d, 1H), 7.689-7.026 (m, Ar H s), 9.744 (s, 1H).
13C NMR (DMSO, 400MHz) (δ/ppm): 153.618, 134.654, 128.89, 126.47, 126.357, 123.525, 117.871, 109.499, 151.832, 134.654, 126.357, 151.832, 147.201, 56.130, 114.442 and 109.34.
GC-MS (m/z): 415.1407 (M+), 301.08443, 279.10241 (base peak), 247.07607, 219.08113, 208.00493, 175.97863 and 151.09348.
Product B
UV-VIS: λmax = 1234 nm (64.018%)
IR (KBr) (υmax/cm-1): 3064, 3032, 2976, 2947 and 2848 (Ar-C-H str), 856, 819,779 (out of plane Ar-C-H bending), 1587 and 1512 (Ar-C=C str), 3365.78 and 3296 (-N-H str), 1587 and 1512 (out of plane –N-H bending), 3498-3033 (broad, hydrogen bonded –O-H str and –N-H str), 1292 and 1269 (-C-O-C str), 1377 (-C-O str), 1664 (-C=O str).
1H NMR (CDCl3, 400 MHz) (δ/ppm): 3.5 (s, 3H), 2.5 (s, 1H), 7. 088-7.082 (d, 1H), 7.689-7.026 (m, Ar H s), 9.783 (s, 1H).
13C NMR (DMSO, 400MHz) (δ/ppm): 55.563, 108.604, 110.695, 115.369, 118.559, 122.604, 125.935, 126.074, 127.494, 153.009, 134.554, 128.678, 167.898, 153.009, 148.137, 153.009 and 155.219.
GC-MS (m/z): 398.0802 (M+), 387.08802, 376.13178, 353.12717, 301.08449, 279.10246, 247.07602, 232.07394, 219.08174, 175.97959, 167.07083 and 151.03954 (base peak).
Amidoalkyl naphthols and aminoalkyl naphthols are important compounds in the field of pharmaceutical chemistry. They may act as starting compounds in the synthesis of many valuable drugs which may cure several diseases such as Parkinson’s disease, hypertension etc. Through this study, we successfully synthesized an aminoalkyl naphthol (product A) from vanillin, 2-naphthol, and 4-nitroaniline and an amidoalkyl naphthol (product B) from vanillin, 2-naphthol and benzamide by various green chemical approaches of synthesis such as microwave irradiation, oil bath, hot plate with magnetic stirrer and Grindstone chemistry method using tannic acid as catalyst. The yield of products are compared with products obtained from normal heating of reactants under reflux using solvent and found that, heating in oil bath is the best method to get maximum products. Oil bath affords 90.01% and 92.1% of products. Microwave irradiation is also good for getting products with yield 87 and 89%. Grindstone chemistry method is only applicable in the synthesis of aminoalkyl naphthol. Optimization study of reactants and tannic acid are done. It is found that, for synthesizing both type of compounds with maximum yield, reactants should be in the ratio 1:1:1.3 mmol where the third constituent is 4-nitroaniline or benzamide. 0.02 mmol of tannic acid is effective in the case of formation of product A (aminoalkyl naphthol) and for product B it is 0.03 mmol of tannic acid. The reactants and products are well separated in TLC when the solvent system is a mixture of hexane and ethyl acetate in the ratio 4:1. Products are isolated from crude sample by column chromatography using 1:1 hexane ethyl acetate mixture as eluting solvent. The isolated compounds are tested for their antibacterial activity against Bacillus Subtilis and found that they shows very little effect on the inhibition against bacterial growth rate. Many factors such as structure of compound, concentration of compounds applied, PH conditions of the culture medium, bacterial growth concentration, size of the petri plate, storage conditions etc may affect the inhibition rate. Hence we hope in future studies, these compounds may exhibit higher efficiency of inhibition against this bacterium by varying these factors or they may exhibit inhibition against growth of other bacteria and also anti-fungal activities.
References
- Shen, G.S.; Layer, R.T.; and Mabe R.T.; Drug Discov., Today. 2000, 5, 98-106.
CrossRef
- Damodiran, N.; Panneer Selvam; Paramasivan T.; Perumal,; Tetrahedron Lett 2009, 5474-5478.
CrossRef - Basappa,; Sengottuvelan Murugan,; Chandagirikoppal, V.; Kavitha,; Anurag Purushothaman,; Kottayath, G. Nevin,; Kazuyuki Sugahara,; Kanchugarakoppal, S.; Rangappa,;Cancer Lett 2010, 231-243.
- Aitken,;. Aitken K. M.; University of St. Andrews, St. Andrews, UK, “1, 4-Oxazines and their Benzo Derivatives”, Comprehensive Heterocyclic Chemistry III, 2008, 8, 373-459.
- Srinivas Kantevari,; Srinivasu V.N.; Vuppalapati,; Lingaiah Nagarapu,; Catalysis Communications, 2007, 266, 109.
CrossRef - Selvam, N. P.; and Perumal, P T.; Tetrahedron Letters, 2006,7481-7483.
CrossRef - Deepali, A.; Kotadia,; Saurabh, S.; Soni,; J. Mol. A. Catal: Chem, 2012, 353-354.
- Zare, A.; Hasaninejad,; Salimi Beni, A.; Moosavi-Zare, A.R.; Merajoddin, M.; Kamali, E.; Akbari-Seddigh, M.; Parsee, Z.; Scientia Iranica, 2011, 18, 433-438.
- Ramin Ghorbani-Vaghei,; Seyedeh Mina Malaekehpour,; Cent. Eur. J. Chem., 2010, 8, 1086-1089.
- Abolghasem Davoodnia,; Rahil Mahjoobin,; Niloofar Tavakoli‐Hoseini,; Chin. J. Catal., 2014, 35, 490-495.
CrossRef
- Samad Khaksar,; Roshanak Najafi,; Seyed Mojtaba Ostad,; Mahgol Tajbakhsh,; World App. Sci. J., 2012, 20, 656-660.
- Ali Dorehgiraee,; Hojatollah Khabazzadeh,; Kazem Saidi; ARKIVOC, 2009, 7, 303-310.
- Zahed Karimi-Jaberi,; Hadi Fakhraei,; Bull. Chem. Soc. Ethiop., 2012, 26, 473-478.
- Hassan Moghanian,; Satter Ebrahimi,; 2014, 18, 165-168.
- Rajesh. Singh,; Robitha Duvedi,; Arabian journal of chemistry, 2014.
- Nana V. Shitole,; Balasahe V. Shitole,; Gopal K. Kakdeb,; Murlidhar S. Shingare,; Orbital: The Electronic Journal of Chemistry, 2013, 5.
- Madan Mohan M, C.; Santosh and Radhaiah, A.; Adikavi Nannaya,; University, Int.J.Curr.Microbiol.App.Sci, 2015, 4, 1000-1008.
- Vikas Verma , Kuldeep Singh , Devinder Kumar, Thomas M. Klapotke , Jorg Stierstorfer , Balasubramanian Narasimhan , Asif Khurshid Qazi , Abid Hamid , Sundeep Jaglan,European Journal of Medicinal Chemistry, 2012, 195-202.
CrossRef - Leila Zamani, Kamiar Zomorodian, Bibi Fathemei Mirjalini, Soghra Khabnadideh Journal of Pharmaceutical and Scientific Innovation, 2014
- Siddique Akbar M. K Ansari,Jayprakash M. Sangshetti, Nagnath D. Kokare, Pravin S. Wakte, Devanand V. Shinde, Indian J. Chem. Technol., 2010, 17, 71-73.
- Nourallah Hazeri, Malek Taher Maghsoodlou, Sayyed Mustafa Habibi-khorassani, Jasem Aboonajmi and Mohyeddin Safarzaei, Chem. Sci. Trans., 2013, 2, S330-S336.
- Hamid Reza Shaterian, Hossein Yarahmadi and Majid Ghashang, Bioorg. Med. Chem. Lett., 2008, 33, 788-792.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









