Fabricating an Air-Retaining Surface on outer Surface of Wetsuit by In-Situ Synthesis of Silica Nanoparticles
Manouchehr Rad1, Ramin Khajavi2, Mina Abbasipour3, Siamak Nazemi4 and Amirhosein Berendjchi3
1Department of Mechanical Engineering, South Tehran Branch, Islamic Azad University, Tehran, Iran.
2Department of Textile and Polymer Engineering, South Tehran Branch, Islamic Azad university, Tehran, Iran.
3Department of Textile Engineering, Science and Research Branch, Islamic Azad University, Tehran, Iran.
4Department of Textile Engineering, Yazd Branch, Islamic Azad University, Yazd, Iran.
Corresponding Author E-mail: rad@sharif.edu
DOI : http://dx.doi.org/10.13005/ojc/330313
In this study, it was aimed to fabricate a superhydrophobic layer as an air-retaining surface on a commercial wetsuit to reduce the drag force. Silica nanoparticles (SiNPs) were synthesized in situ on the outer surface of a wetsuit through sol-gel method and then they were treated with hydrolyzed n-octyltriethoxysilane (OTES). Scanning electron microscopy (SEM) and atomic-force microscopy (AFM) images showed a uniform formed coating of SiNPs on the surface of wetsuit. The values of Static water-contact angle (SWC) and water-shedding angle (WSA) for hydrolyzed samples were 153° (for a 10 µL water droplet) and 34° (for a 15 µL water droplet) respectively. Attributed to the desired formed air film, the wind tunnel test results showed drag reduction for coated wetsuit. The introduced product showed high potent for long distance swimming and triathlon.
KEYWORDS:Wetsuit; Drag force; Air retaining surface; Superhydrophobic surface; Air film
Download this article as:| Copy the following to cite this article: Rad M, Khajavi R, Abbasipour M, Nazemi S, Berendjchi A. Fabricating an Air-Retaining Surface on outer Surface of Wetsuit by In-Situ Synthesis of Silica Nanoparticles. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Rad M, Khajavi R, Abbasipour M, Nazemi S, Berendjchi A. Fabricating an Air-Retaining Surface on outer Surface of Wetsuit by In-Situ Synthesis of Silica Nanoparticles. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=34100 |
Introduction
Drag or hydrodynamic resistance plays an important role in swimming performance and in general for every moving object submerged in water. Over 90% of the swimmer’s power output is spent overcoming hydrodynamic drag [1, 2]. There are three different types of drag: pressure, wave, and friction. Pressure or form drag is the energy required to move fluid in front of an object in the flow. The energy required for creating velocity gradients in the fluid layer to move the object is named drag [3]. The speed of swimmers depends on hydrodynamic drag force. To achieve higher speed, the hydrodynamic drag force should be reduced for forward motion. Several studies have investigated the effect of swimsuits on hydrodynamic drag [4-7].
One way to reduce drag is to use a layer of air bubbles on the surface of the object. The air‑water interface reduces the hydrodynamic force. In this field, superhydrophobe surfaces play an important role, as they are capable of trapping air pockets due to their roughness and their low surface energy because of micro and nanoscale features. A superhydrophobic substrate is defined as a surface with low wettability and with a contact angle (CA) greater than 150°. Many fabricated superhydrophobic surfaces are based on mimicking biological air-retaining plants such as the floating ferns Salvinia and the backswimmer Notonecta. The attempt to reduce hydrodynamic drag was originally inspired by the unique properties of fast-swimming shark skin. A layer of air trapped on the surface of the object leads to slip which exhibits drag reduction. This slip reduces drag at higher Reynolds numbers in both laminar and turbulent regimes [8, 9]. Different studies presented the drag reduction of laminar flow through microchannels by using hydrophobic surfaces [10-12]. For example, Henoch et al. (2006) [13] demonstrated that superhydrophobic surfaces reduce the drag force at velocities up to 1.4 m/s.
Up to now many methods have been presented for fabricating such surfaces on different substrates, [14-17] including lithography, chemical vapour deposition, a sol-gel process, electrochemical deposition, layer-by-layer assembly, and etching [18-22]. A sol-gel process is a wet chemical procedure that makes it simple to synthesize metal-oxide nanoparticles. In this process, metal alkoxide, which is used as a precursor, is dissolved in water or alcohol to form a colloidal solution (sol). The gel is formed through hydrolyses and then condensation of the colloidal solution. The sol-gel method is economical—it, as a low-temperature technique, affords good control of the obtained products. Typically, silicon alkoxide groups, such as tetraethoxysilane Si(OC2H5)4 (TEOS), have been used as Si precursors [23-29].
Swimwear can be divided into two categories: wetsuits and drysuits. Both types of diving suits protect divers from water and the elements. Drysuits are mostly used for warm water. Wetsuits show some benefits over drysuits including being relatively inexpensive and easily wearable. It is usually made of foamed neoprene coated sometimes with a fabric such as PET. It is used by surfers, divers, windsurfers, canoeists, and others engaged in water sports, providing thermal insulation, abrasion resistance, and buoyancy. Bubbles of gas in the surface of wetsuits lead to drag reduction as well as heat conduction. They make the wetsuit with a low density and provide buoyancy in water. Its drag or hydrodynamic resistance depends on the behaviour of the outer surface.
By considering different literatures, there is no study to reduce the drag of wetsuits by superhydrophobic coating to ease body movement. So in this study, it was intended to convert the outer surface of the wetsuit into an air-retaining surface by fabricating a superhydrophobic surface on it and thereby reducing the hydrodynamic drag. SiNPs were synthesized in-situ on a commercial wetsuit by sol-gel method. They were treated with a hydrolyzed alkyl silane solution. Samples were characterized by scanning electron microscopy (SEM), atomic-force microscopy (AFM), and energy-dispersive spectroscopy (EDS). The superhydrophobic effect was investigated by measuring the SWC, WSA, and surface energy. Finally, the hydrodynamic drag of samples determined in a wind tunnel and compared with untreated samples.
Materials and Methods
A commercial wetsuit (including PET fabric coated with neoprene) provided by Mares Company (Rapallo, Italy). Tetra ethyl ortho silicate (TEOS), n-octyltriethoxysilane (OTES), ammonium hydroxide (25%), ethanol (96%) and hydrochloric acid were purchased from Merck.
Procedures
SiO2 nanoparticles were synthesized in accordance with the Stöber method [30]. Briefly, ethanol (25 ml), ammonium hydroxide (1 ml), distilled water (3.6 ml), and TEOS (11.5 ml) were stirred for 2 h at room temperature to prepare the sol-gel solution. Wetsuit samples (5 cm2) were immersed in the prepared solution at 30°C for 5 min, dried at 60°C, and then cured at 160°C for 5 min. OTES (17 ml) and hydrochloric acid (0.05 M, 4.2 ml) were mixed and stirred for 24 h at room temperature. Samples were immersed in hydrolyzed and diluted OTES (with 4% wt/wt ethanol) for 4 h at room temperature. The samples treated with hydrolyzed OTES were cured at 120°C for 1 h [30].
Characterization
The morphology of samples was observed using SEM (XL30, Philips, Royal Philips Electronics, Amsterdam, Netherlands), and their surface roughness was measured via AFM (Danish Micro Engineering A/S, Copenhagen, Denmark) in tapping mode. Fourier Transform Infrared (FTIR) spectra were measured by FTIR spectrophotometer (Bruker Optics) in ATR mode (Germany). The SWC was measured using a contact-angle measurement device (Data physics OCA, Germany). A 10 μl droplet of deionized water was placed at each of the five different positions on the sample surfaces, and the angle of each drop on the sample was determined. The SWC values and surface energy of the samples were reported based on an average of three measurements (Dataphysics OCA, Germany). The WSAs of the various samples were measured following the method of Zimmermann et al. [31]. The droplet (15 μl) was released at a height of 1cm, and the minimum angle at which the droplet started to roll off the surface was then determined. Each test was repeated three times.
The drag reduction was studied by wind tunnel. The experiments were performed in a closed-circuit wind tunnel of 45 × 45 cm2 test section with a length of 120 cm at Amirkabir University of Technology. The experimental setup is shown in Figure 1. A cylinder of 110 mm diameter and 200 mm length was covered with fabric and stood at an angle of 90°. The wind tunnel was run at a velocity of 15 m/s.
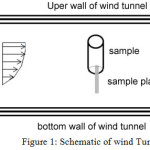 |
Figure 1: Schematic of wind Tunnel |
Results and Discussion
The process for synthesizing SiNPs is shown in Figure 2. TEOS was hydrolyzed and silica nanoparticles nucleates were obtained by Si-O-Si bonding during the condensation reaction; thereby, the sol-gel solution was obtained. The surface of the solvent was evaporated after the sample treated with the sol solution had been dried and cured. In this step, the treated sample was still hydrophilic due to the presence of hydroxyl groups in the silicon nanostructures. The hydrophobic surface was then obtained by the interaction of hydrolyzed OTES with silanol groups (Figure 2) [31].
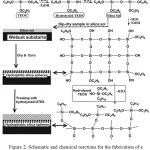 |
Figure 2: Schematic and chemical reactions for the fabrication of a superhydrophoic SINPs on the surface of a wetsuit. Click here to View figure |
The surface morphologies of the untreated and OTES-TEOS-treated samples were investigated by electron microscopy. The SEM images of the samples are shown in Figure 3. As shown in the Figure 3a, the untreated sample had a smooth structure, whereas the treated sample with SiNPs had a rough surface. An SEM survey of 20 synthesized SiO2 nanoparticles showed that the fabricated nanoparticles had an average size of 76 nm.
 |
Figure 3: SEM images of (a) Untreated, (b) TEOS and (c) OTES−TEOS coated samples, and (d) Particles size of SiO2 nanoparticles for OTES−TEOS coated samples. Images on the right are at higher magnification. Click here to View figure |
The AFM images of the untreated and OTES−TEOS-treated wetsuit surface are provided in Figure 4. It is obvious that the untreated sample is smooth (Figure 4a), while the treated sample has a rough surface (Figure 4b). The root-mean-square (RMS) roughness of the wetsuit was also measured by AFM-software. The untreated sample had an RMS roughness of 46 ± 0.05 nm, whereas the treated wetsuit samples with OTES-TEOS had an RMS roughness of 80±0.1 nm.
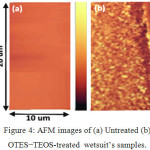 |
Figure 4: AFM images of (a) Untreated (b) OTES−TEOS-treated wetsuit’s samples. |
The surface elemental analysis of the treated wetsuit was carried by EDS (Table 1). The results show just an Au peak for the untreated sample, while there is a Si value for both TEOS and TEOS-OTES samples. As expected, the amount of Si increased from 65.43% to 92.99% for TEOS-OTES samples. Therefore, post treatment of TEOS samples with OTES result in to more deposition of Si on the surface.
To consider the characteristic peaks of silicon and its oxide form, the FTIR transmittance spectra of different samples are shown in Figure 5 in the range of 750 cm -1 to 1250 cm -1. The characteristic bands at 800 cm -1 and 900 cm -1 are attributed to the stretching of Si‑O bending and Si‑OH bonds respectively. The characteristic bond at 1090 cm -1 is related to the stretching of Si‑O‑Si. It is seen that there are no Si characteristic bonds for untreated samples. On the other hand, TEOS-OTES sample has weaker Si‑OH stretching intensity bond (930 cm-1) than TEOS sample. It is a confirmation for the reduction of Si‑OH bonds after treating TEOS sample with hydrolyzed alkyl silane (Hydrolysed OTES) [32-33].
Table 1: Energy Dispersive X-ray Spectroscopy results of a) pristine polyester b) TEOS-treated sample c) OTES−TEOS-treated sample
| Sample Kind / treated sample with | |||
| Element | Pristine (%) | TEOS (%) | TEOS-OTES (%) |
| Si | – | 65.43 | 92.99 |
| Au | 100% | 34.57 | 7.01 |
| Total | 100 | 100 | 100 |
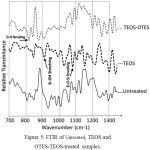 |
Figure 5: FTIR of Untreated, TEOS and OTES-TEOS-treated samples. |
The water-repellent properties of untreated, TEOS-treated, and OTES-TEOS-treated samples are presented in Figure 6. On the untreated sample, the water droplet does not distribute and penetrates the surface in the same place. On the OTES-TEOS-treated sample, the water droplet gets distributed on the surface and does not penetrate, because the pores are filled with SiO2 nanoparticles.
 |
Figure 6: Contact angles and wettability of (a) Untreated, (b) TEOS and(c) OTES−TEOS-treated samples |
The contact angle of water droplets on untreated, TEOS-treated, and OTES-TEOS-treated samples are shown in Figure 6. The water-contact angle on the TEOS-treated sample (76± 0.5°) is lower compared to other samples (Figure 6b & Table 2). It is because of the presence of hydroxyl groups following the TEOS treatment (Figure 2). Figure 6c shows a spherical-like water droplet with a contact angle of 153± 1.5° (Table 2). The WSA on the OTES-TEOS-treated sample has decreased to 30°. The surface energy of the OTES-TEOS-treated sample is significantly lower than that of the TEOS-treated sample due to the presence of hydroxyl groups in the latter.
Table 2: Static water, water shedding angles and surface energy of pristine, TEOS and OTES−TEOS-treated samples.
| Sample kind / treated with | Mean of static contact angle “SWC” (°) | Mean of waster shedding angle “WSA” (°) | Surface Energy (N/m) |
| Pristine | 109± 1.5 | 54±0.1 | 34±2.7 |
| TEOS | 76±0.5 | 63±0.3 | 123±3.2 |
| OTES−TEOS | 153±1.5 | 30±0.3 | 4.5±0.05 |
The surface roughness of a swimsuit or any other moving object submerged in water has an important role in the resultant drag force. In this paper, the drag coefficient (CD) is presented as a function of Reynolds number (Re) (Figure 7). The CD and Re were calculated by using the following formulas:
CD= FD /0.5ρV2A (1)
Where FD is drag force, ρ density, V velocity of wind, and A area of samples.
Re= ρVL/μ (2)
Where ρ, V and μ are density, velocity, and viscosity of wind and L is sample length (Marhino et al., 2012).
For comparison, the CD values of the bare cylinder are also presented in the figure. The surface roughness has a significant effect on the air flow characteristics over the surface.
The data indicates the CD start to decrease for untreated sample at Re=1.2 × 105. Similarly, the CD of OTES-TEOS samples reduced at Re=1.5 × 105. It is because of the surface tension decreased by introducing OTES. Moreover, superhydrophobic surfaces contain nanoscale features which cause air trapping and slip. Therefore, the slip leads to drag reduction at higher Reynolds numbers in both laminar and turbulent regimes (Dean & Bhushan, 2010). In contrast, due to increasing surface tension, the TEOS sample undergoes increase at Re=2 × 105.
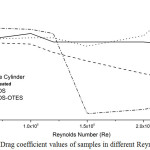 |
Figure 7: Drag coefficient values of samples in different Reynolds numbers Click here to View figure |
Conclusion
A commercial wetsuit was coated with hydrophobic silica nanoparticles by the sol-gel method. The fabricated layer produced a superhydrphobic layer on the outer layer of the wetsuit due to the produced roughness and the low energy of the surface. The wind test results showed the reduction of hydrodynamic drag for the coated wetsuit—especially in high Reynolds numbers. It indicates that the superhydrophobic-fabricated layer can act as an air-retaining surface and so the produced air film can reduce the drag due to slip phenomena. The modified wetsuit shows high potential for maximizing the mobility of limbs and can be used for distance swimming and triathlon. It can also cooperate with the enclosed bubbled gas of neoprene for improving the insulating ability of such a wetsuit.
References
- Moria, H.; Chowdhury, H.; Alam, F.; Subic, A. Journal of mechanical and industrial engineering. 2011, 5, 83-87.
- Moria, H.; Chowdhury, H.; Alam, F. Procedia Engineering. 2011, 13, 389–394.
CrossRef - Dean, B.; Bhushan, B. Phil. Trans. R. Soc. A. 2010, 368, 4775–4806.
CrossRef - Marinho, D. A.; Mantha, V. R.; Vilas-Boas, J. P.; Ramos, R. J.; Machado, L.; Rouboa, A. I.; Silva, A. J. Brazilian archives of biology and technology. 2012, 55, 851-856.
CrossRef - Marinho, D. A.; Mantha, V. R.; Rouboa , A. I.; Vilas-Boas, J. P.; Machado, L.; Barbosa, T. M., Silva, A. J. Port J Sports Sci. 2011, 11, 319-322.
- Mollendorf, J. C.; Termin, L. A. C.; Oppenheim, E.; Pendergast, D. R. Med Sci Sports Exerc. 2004, 36, 1029-1035.
CrossRef - Roberts, B. S.; Kamel, K. S.; Hedrick, C.E.; Mclean, Sharp, R. L. Med Sci Sports Exerc. 2003, 35, 519-524.
CrossRef - Bhushan, B. Langmuir. 2012, 28, 1698−1714.
CrossRef - Bixler, G. D.; Bhushan, B. Soft Matter. 2012, 8, 11271-11284.
CrossRef - Daniello, R. J.; Waterhouse, N. E.; Rothstein, J. P. Physics of fluids. 2009, 085103-1-9.
- Kim, Y. W.; Lee, J. M.; Lee, I.; Lee, S. H.; Ko, J. S. International journal of precision engineering and manufacturing, 2013, 14, 299-306.
CrossRef - Moaven, Kh.; Rad, M.; Taeibi-Rahni, M. Experimental Thermal and Fluid Science. 2013, 51, 239–243.
CrossRef - Henoch, C., Krupenkin, T., Kolodner, P., Taylor, J., Hodes, M., Lyons, A., Breuer, K. Turbulent drag reduction using superhydrophobic surfaces. 3rd AIAA Flow Control Conference. 2006.
CrossRef - Krupenkin, T. N.; Kolodner, P.; Taylor, J. A.; Hodes, M. S.; Lyons, A. M.; Peguero, C.; Breuer, K. Turbulent Drag Reduction Using Superhydrophobic Surfaces, 3rd AIAA Flow Control Conference 5 – 8 June 2006, San Francisco, California.
- Zhang, Y. L.; Xia, H.; Kim, E.; Sun, H. B. Soft Matter, 2012, 8, 11217-1123.
CrossRef - Shirtcliffe, N. J.; McHale, G.; Atherton, S.; Newton, M. I. Adv. Colloid Interface Sci. 2010, 161, 124-138.
CrossRef - Yao, X.; Song, Y.; Jiang, L. Adv. Mater. 2011, 23, 719-734.
CrossRef - Ionov, L.; Synytska, A. Phys. Chem. Chem. Phys., 2012, 14, 10497-10502.
CrossRef - Ma, M.; Mao, Y.; Gupta, M.; Gleason, K. K.; Rutledge, G. C. Macromolecules, 2005, 38, 9742-9748.
CrossRef - Shi, J.; Alves, N. M.; Mano, J. F. Bioinspiration&Biomimetics, 2008, 3, 1-6.
CrossRef - Han, D.; Steckl, A. J. Langmuir, 2009, 25, 9454-9462.
CrossRef - Pozzato, A.; Dal Zilio, S., Fois, G.; Vendramin, D.; Mistura, G.; Belotti, M.; Chen, Y.; Natali, M. Microelectronic Engineering, 2006, 83 (2006), 884-888.
CrossRef - Bae, G. Y.; Min, B. G.; Jeong, Y. G.; Lee, S. C.; Jang, J. H.; Koo, G. H. J Colloid Interface Sci. 2009, 337, 170-175.
CrossRef - Gao, Q.; Zhu, Q.; Guo, Y. Ind. Eng. Chem. Res., 2009, 48 9797-9803.
CrossRef - Hao, L. F.; An, Q. F.; Xu, W.; Wang, Q. J. Adv. Mater. Res. 2010, 121, 23-26.
CrossRef - Li, Z.; Xing, Y.; Dai, J. Appl. Surf. Sci. 2008, 254, 2131-2135.
CrossRef - Yu, M.; Gu, G.; Meng, W. D.; Qing, F. L. Appl. Surf. Sci. 2007, 253, 3669-3673.
CrossRef - Xue, C. H.; Jia, S. T.; Zhang, J.; Tian, L. Q. Thin Solid Films. 2009, 517, 4593-4598.
CrossRef - Xu, B.; Cai, Z.; Wang, W.; Ge, F. Surf. Coat. Technol. 2010, 204, 1556-1561.
CrossRef - Brendjchi, A.; Khajavi, R.; Yazdanshenas, M. E. Nanoscale research letters. 2011, 6, 594-601.
CrossRef - Zimmermann, J.; Seeger, S.; Reifler, F. A. Text. Res. J, 2009, 79, 1565-1570.
CrossRef - Bange, J. P.; Patil, L. S.; Gautam, D. K. Progress In Electromagnetics Research M, 2008, 3, 165–175, 2008.
CrossRef - Shokri, B.; Abbasi Firouzjah, M.; Hosseini, S. FTIR analysis of silicone dioxide thin film deposited by metal organic-based PECVD, International plasma chemistry society (ISPC19), 2009, Bochum, Germany.

This work is licensed under a Creative Commons Attribution 4.0 International License.









