Development of the Composition and Method of Producing a Liquid Complex Fertilizers with a Stabilizing Additive
U. B. Nazarbek1, A. A. Kadirbayeva1, M. Zh. Aitureev2, O. P.Bayisbai1 and L. Aikozova3
1Department of Chemical Technology of Inorganic Substances, M.Auezov South Kazakhstan State University, Shymkent, Kazakhstan.
2Department of Chemical technology of silicate and refractory non-metallic substances. M.Auezov South Kazakhstan State University, Shymkent, Kazakhstan.
3Department of Chemistry. M.Auezov South Kazakhstan State University, Shymkent, Kazakhstan.
Corresponding Author E-mail: eplusr@bk.ru
DOI : http://dx.doi.org/10.13005/ojc/330335
The article is devoted to the study of technological bases of processing of phosphate waste – cottrell dust and vermiculite as a stabilizing additive in a liquid complex fertilizer. Target of the research work were implemented as a result of engaging in technological conversion of solid industrial waste – cottrell dust. While cottrell dust was used as a phosphorus raw material, and vermiculite as the substrate, for mulching and aeration of the soil, nourishes the plants with minerals. Results of studies allowed to establish as a optimal regime parameters of the process of obtaining liquid complex fertilizers.
KEYWORDS:chemical industry; cottrell dust; recycling; industrial waste; liquid complex fertilizer
Download this article as:| Copy the following to cite this article: Nazarbek U. B, Kadirbayeva A. A, Aitureev M. Z, Bayisbai O. P, Aikozova L. Development of the Composition and Method of Producing a Liquid Complex Fertilizers with a Stabilizing Additive. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Nazarbek U. B, Kadirbayeva A. A, Aitureev M. Z, Bayisbai O. P, Aikozova L. Development of the Composition and Method of Producing a Liquid Complex Fertilizers with a Stabilizing Additive. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33485 |
Introduction
Liquid fertilizers are water solutions or slurries of mineral and some organic nutrients. The most common mineral nitrogen – and phosphorus-containing liquid complex fertilizers. This product group includes solutions of salts containing two or three major nutrients (N, P, K) and macronutrients (Ca, Mg, S) and micronutrients (Fe, Mn, B, Cu, Zn, Mo, Co). Nitrogen-phosphorus liquid complex fertilizer are solutions of phosphates of ammonium, obtained by high temperature treatment with ammonia, H3PO4 or polyphosphoric acids [1].
Material and Methods
Unlike the traditional methods, for the production of housing services uses a mixture of cottrell dust, vermiculite and humic acid at [2] various ratios. Vermiculite of Kulantau field [3] having the following composition: SiO2– 37,44, СаО-2,10, MgO-23,88, K2O+Na2O-1,18, Fe2O3 – 6,01, Al2O3 – 11,23, Н2О – 17,18, used as stabilizing additive [4]. Pre-synthesized humic acid with pH = 0,760, as in previous works, is used as a substitute for sulphuric, nitric or phosphoric acids.
It was established that the composition cottrell dust [5] meets the following content, %: P2O5 total – 25, K2O – 5, Na2O- 1, SiO2 – 25, CaO – 10, MgO – 1, Al2O3 – 3, Fe2O3 – 1, C – 24.
A mixture of finely cottrell dust, activated water, vermiculite, of a dilution solution consisting of potassium sulphate and 30% ammonium sulphate is decomposed by humic acid at a temperature of 600C and 90 minutes. The addition of solutions of potassium sulphate and ammonium sulphate increases [6] potassium and nitrogen and consequently in the composition of the liquid complex fertilizer [7].
The process chemistry is possible will present as follows [8,9]:
Ca5(PO4)3F + 6Humic Acid + 2(NH4) 2SO4 + К 2SO4 + Н2О = 2CaSO4 + NH4(Hum) + H3PO4 + 2Са(Hum)2+ Ca(H2PO4)2 + HF + 2КHum
Process flow diagram for synthesizing the liquid complex fertilizer is shown in Fig. 1.
The mixture after decomposition is filtered. It is established that the obtained liquid phase has a density of 1.2 and pH = 2,7.
The resulting solution is analyzed for the content of assimilable and water-soluble P2O5, ammonia and nitrogen.
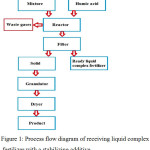 |
Figure 1: Process flow diagram of receiving liquid complex fertilizer with a stabilizing additive. Click here to View figure |
Results and discussion
Laboratory work has been tested in the laboratory of the Department Chemical technology of inorganic substances, M. Auezov SKSU, and physico-chemical analyses [10] were performed in the Regional Laboratory Test Engineering Profile (RLTEP) on the basis of the M. Auezov SKSU.
The resulting product has the following material composition:
Table 1: Physico – chemical parameters obtained in the laboratory liquid complex fertilizer.
| № | Name of indicators | Indicators |
| 1 | Mass fraction of total phosphates, % | 11,3 |
| 2 | Mass fraction of assimilable phosphates, % | 11,0 |
| 3 | Mass fraction of total nitrogen (N), % | 0,75 |
Physico-chemical features derived from cottrell dust liquid complex fertilizer. Using a scanning electron microscope is performed element-by-element and mineralogical analysis of its composition. The results of the microscopic studies allowed to image the surface of samples and the spectra of individual points, with the idea element by element, and percentage composition, as well as diffraction peaks of individual elements with high spatial resolution and the desired depth of field of view.
Microscopic picture and the results of elemental analysis of the liquid complex fertilizer is presented in fig.2 and table 2.
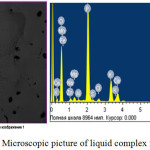 |
Figure 2: Microscopic picture of liquid complex fertilizers. Click here to View figure |
Table 2: Element wise composition of liquid complex fertilizer, calcined at 5000C.
| Element | Weight composition, % | Weight composition, in terms of oxide, % |
| O | 54,07 | – |
| Na | 1,38 | 1,86 |
| Mg | 1,30 | 2,15 |
| Al | 0,31 | 0,58 |
| P | 27,52 | 63,04 |
| S | 0,48 | 1,20 |
| Cl | 0,98 | – |
| K | 9,16 | 11,95 |
| Ca | 3,99 | 5,58 |
| Mn | 0,22 | 0,28 |
| Zn | 0,49 | 0,60 |
| Fe | 0,10 | 0,12 |
The dry residue remaining after filtration is also is a useful product which corresponds to a double super phosphate low grade (Fig.3, table 3). The residue is granulated and dried at a temperature of 100-1100С in 3-5 hours. The obtained dry residue has the following physical characteristics, %: P2O5 total – 20; P2O5 assimilable-15; P2O5 water-soluble-8,75.
The dry residue remaining after filtration is also is a useful product which corresponds to a double super phosphate low grade (Fig.3, table 3). The residue is granulated and dried at a temperature of 100-1100С in 3-5 hours. The obtained dry residue has the following physical characteristics, %: P2O5 total – 20; P2O5 assimilable-15; P2O5 water-soluble-8,75.
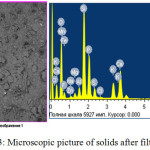 |
Figure 3: Microscopic picture of solids after filtration. Click here to View figure |
Table 3: Element wise composition of solids.
| Element | Weight composition, % | Weight composition, in terms of oxide, % |
| O | 43,95 | – |
| Na | 1,48 | 1,99 |
| Mg | 1,98 | 3,28 |
| АІ | 2,77 | 5,23 |
| Si | 19,49 | 41,68 |
| P | 10,96 | 25,19 |
| S | 2,28 | 5,70 |
| Cl | 0,52 | – |
| K | 4,85 | 6,32 |
| Ca | 9,11 | 12,74 |
| Mn | 0,24 | 0,30 |
| Zn | 0,86 | 1,06 |
| Ғе | 0,53 | 0,67 |
IR spectral analysis of liquid complex fertilizers was conducted on the instrument IR spectrometer Shimadzu IR Prestige-21. In table 4 and fig.4 shows the main peaks according to the results of IR spectral analysis of liquid complex fertilizers.
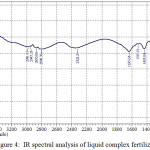 |
Figure 4: IR spectral analysis of liquid complex fertilizers. Click here to View figure |
Table 4: The peaks in the IR-spectral analysis of liquid complex fertilizers.
|
No. |
Peak |
Intensity |
Corr. intensity |
Base (H) |
Base (L) |
Area |
Corr. area |
|
1 |
551,64 |
67,192 |
0,980 |
574,79 |
547,78 |
4,367 |
0,098 |
|
2 |
605,65 |
65,697 |
2,139 |
613,36 |
578,64 |
5,902 |
0,385 |
|
3 |
875,68 |
29,990 |
2,608 |
891,11 |
617,22 |
98,804 |
4,773 |
|
4 |
925,83 |
27,691 |
6,704 |
1014,56 |
894,97 |
56,342 |
4,823 |
|
5 |
1041,56 |
43,898 |
3,995 |
1103,28 |
1018,41 |
27,792 |
1,816 |
|
6 |
1134,14 |
51,955 |
5,310 |
1365,60 |
1107,14 |
43,230 |
2,061 |
|
7 |
1435,04 |
78,933 |
6,091 |
1496,76 |
1381,03 |
9,916 |
1,740 |
|
8 |
1519,91 |
83,358 |
0,458 |
1557,62 |
1500,62 |
2,100 |
0,040 |
|
9 |
1635,64 |
76,504 |
8,317 |
1932,67 |
1531,48 |
33,421 |
6,617 |
|
10 |
2322,29 |
79,715 |
0,201 |
2330,01 |
1978,97 |
26,267 |
0,041 |
|
11 |
2808,36 |
78,723 |
2,333 |
2846,93 |
2549,89 |
26,765 |
2,349 |
|
12 |
2870,08 |
79,866 |
1,492 |
2916,37 |
2850,79 |
6,201 |
0,459 |
|
13 |
2947,23 |
81,359 |
0,461 |
2954,95 |
2920,23 |
2,994 |
0,063 |
|
14 |
2981,95 |
80,959 |
0,879 |
3151,69 |
2958,80 |
16,234 |
0,590 |
IR spectra of the studied [10] samples of the liquid complex fertilizers are characterized by intense peaks of the broad bands of absorption with satellites in the areas of 500-900 cm-1 and 2600-3700 cm-1. It should be noted that the wavelengths in the absorption region of 550-700 cm-1 is characteristic for alkanes and alkene with communication RC≡CH, and 850-900 cm-1 for compounds alkane and alkene of a number of types of the C=C=C. The peaks of intensive absorption in the region of 2600-3700 cm-1 characterizes carbonyl group aromatic a number of types CH2-COOH, C=C-COOH.
In table 5 and fig.5 shows the main peaks according to the results of infrared spectral analysis of dry residue after filtration.
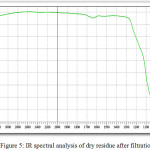 |
Figure 5: IR spectral analysis of dry residue after filtration. Click here to View figure |
Table 5: The peaks in the IR-spectral analysis of dry residue after filtration
|
No. |
Peak |
Intensity |
Corr. intensity |
Base (H) |
Base (L) |
Area |
Corr. area |
|
1 |
563,21 |
69,162 |
3,293 |
578,64 |
547,78 |
4,671 |
0,336 |
|
2 |
601,79 |
70,888 |
5,015 |
621,08 |
582,50 |
5,134 |
0,450 |
|
3 |
655,80 |
77,998 |
0,153 |
659,66 |
640,37 |
2,057 |
0,013 |
|
4 |
675,09 |
77,475 |
0,115 |
678,94 |
659,66 |
2,104 |
0,004 |
|
5 |
802,39 |
68,978 |
4,538 |
840,96 |
686,66 |
20,773 |
1,387 |
|
6 |
910,40 |
66,648 |
3,849 |
956,69 |
844,82 |
18,315 |
1,522 |
|
7 |
1033,85 |
42,840 |
31,505 |
1330,88 |
960,55 |
59,024 |
25,826 |
From fig.5 and table 5 data follows:
– 1049-1060 absorption spectra with wavelengths characteristic of phosphorus compounds, P=O (with hydrogen bonds);
– 952-906 typical P-F and phosphates;
– absorption spectra with wavelengths of 1020-1090 characterize the presence of cottrell dust in compounds of silicates with valence relations Si-O-Si;
– absorption spectra in the region 800-802 characteristic of silicate compounds charge materials in the valence state of Si-O-Ca and Si-O-Al [10];
Studied optimal regime parameters to the production of liquid complex fertilizers. The results are shown in table 6 and fig.6.
Table 6: The results of the research of optimal regime parameters.
|
Time, min |
Р2О5 total, % |
Р2О5 assimilable, % |
N, % |
|
1 |
2 |
3 |
4 |
|
300С |
|||
|
90 |
9,5 |
9,0 |
0,5 |
|
60 |
9,4 |
8,36 |
0,5 |
|
30 |
9,0 |
7,76 |
0,12 |
|
400С |
|||
|
90 |
9,75 |
9,0 |
1,0 |
|
60 |
9,6 |
8,75 |
0,75 |
|
30 |
9,5 |
8,5 |
0,75 |
|
600С |
|||
|
90 |
11,3 |
11,0 |
0,75 |
|
60 |
11,0 |
10,75 |
0,75 |
|
30 |
11,0 |
10,75 |
0,5 |
|
750С |
|||
|
90 |
10,3 |
10,0 |
0,5 |
|
60 |
10,3 |
9,8 |
0,5 |
|
30 |
10,0 |
9,75 |
0,3 |
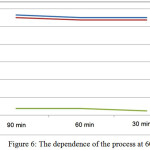 |
Figure 6: The dependence of the process at 600C. Click here to View figure |
From table 3.2 it is seen that the optimal regime indicators developed technology for production of liquid complex fertilizers are:
– temperature – 600C;
– for the duration of the process – 90 min.
Conclusion
Thus, on the basis of a mixture of phosphorus sludge and vermiculite in conditions of temperature 60 degrees and at optimum process duration of 90 minutes it is possible to obtain liquid complex fertilizer with the content of assimilable phosphoric anhydride – 11%, nitrogen is 0.75%, zinc is 0.60%; the dry residue obtained after the stage filtration, is composed of : phosphor – of 10.96% (calculated as P2O5 – 25%, including Р2О5 assimilable – 15%, P2O5 water-soluble – 8,75%), in line with double superphosphate; the source of the vermiculite used in the process of decomposition of the phosphate of secondary raw materials, is a highly effective stabilizing additive, which are used to improve soil structure, referred to as the “agronomic” rock. The residual part of the utility and double superphosphate humic acid will fulfill the role of organic-mineral additives, which improve the soil structure, and show the quality of the protective compositions of plants from pests and diseases.
References
- Kapranov, V.N., The use of natural fertilizers and mineral nutrition of field crops. Author’s abstract of Doctoral thesis. Moscow, 2009.
- Nazarbek, U.B.; Besterekov, U; Petropavlovsky, I.A.; Nazarbekova, S.P.; Beisenbayev, O.K., Orien. J. Chem., 2015, 31(3), 1409-1416.
- The field of mining raw materials of Kazakhstan: guide. Almaty, 2000.
- Ivanova, L.A.; Kotelnikov, V.V.; Bykova, A.E., Physico-chemical transformation of the mineral vermiculite into the substrate for growing plants. Bulletin of MSTU, 2006, 9, 885-891.
- Nazarbek, U.B.; Besterekov, U; Nazarbekova, S.P.; Bolysbek A.A., Orien. J. Chem., 2015, 31(1), 215-221.
- Ulianova, O.A., Ecological and agrochemical assessment of fertilizer compositions to increase the productivity of the system soil-plant. Author’s abstract of Doctoral thesis. Ulan-Ude, 2011.
- Bolatbekuly, O.; Aitureyev, M.; Nazarbek, U.B., Bulletin of KazNRTU, 2016, 6(118), 541-545.
- Nazarbek, U.B.; Besterekov, U; Nazarbekova, S.P.; Beisenbayev, O.K.; Bolysbek A.A., Orien. J. Chem., 2016, 32(4), 2027-2033.
- Besterekov, U; Nurasheva, K.K.; Nazarbek, U.B.; Nazarbekova, S.P.; Bolysbek A.A., Orien. J. Chem., 2017, 33(1), 92-103.
- Nakhmanson, M.S.; Feklichova, V.G., Diagnosis of the composition of materials x-ray diffraction and spectral methods. Leningrad, 1990.

This work is licensed under a Creative Commons Attribution 4.0 International License.









