An Insight Into Antibacterial and Anticancer Activity of Homo and Hetero Binuclear Schiff Base Complexes
Bhoopathy Parasuraman, Jayalakshmi Rajendran and Rajavel Rangappan
Department of Chemistry, Periyar University, Salem-636 011, Tamilnadu, India.
Corresponding Author E-mail: drrajavelpu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330321
Mononuclear complex has been used as a building block in the synthesis of homo/hetero binuclear complexes. The complex behaves as a potential metallo-ligand. Its reaction with different metal salts (Ni & Cu) lead to the formation of binuclear complexes. The homo and hetero binuclear oxygen bridged Cu(II) and Ni(II) complexes had been synthesized from the Schiff base ligand derived from 4-chloro-o-phenylenediamine and 3,5-dichloro-2-hydroxyacetophenone. The synthesized complexes have been characterized with the support of more than a few standard physicochemical techniques such as elemental analysis, spectroscopic, thermal, cyclic voltammetry and magnetic moment studies. Spectral analysis exhibits square planer geometry for Cu(II) & Ni(II) mono and binuclear complexes. The antimicrobial results indicate that the homo-binuclear copper complex exhibit more activity than the other mono and binuclear complexes. The Schiff base and their complexes have been screened for their anticancer activity using MCF7 cell line.
KEYWORDS:4-chloro-o-phenylenediamine; thermal; antibacterial; MCF7; square planar
Download this article as:| Copy the following to cite this article: Parasuraman B, Rajendran J, Rangappan R. An Insight Into Antibacterial and Anticancer Activity of Homo and Hetero Binuclear Schiff Base Complexes. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Parasuraman B, Rajendran J, Rangappan R. An Insight Into Antibacterial and Anticancer Activity of Homo and Hetero Binuclear Schiff Base Complexes. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=32837 |
Introduction
The interaction of organic / inorganic ligands with the metal centers is one of the most active research areas in inorganic chemistry. Coordination chemistry includes different types of coordination complexes applicable in a wide diversity of fields such as, catalysis, bioinorganic chemistry, medicine, ceramics, material science and toxicology. However, significant increasing drug resistance has limited the clinical applications of metal compounds activity and biological applications including antimicrobial, insecticidal, anti-HIV, antitumor and in vitro-cytotoxic activities as well as DNA binders. Transition metal ions such as Pt(II), Cd(II), Hg(II) and Pb(II) are recognized as highly toxic, which makes their presence in environmental waters or soils disagreeable. Even at very low concentrations such metals produces toxic effects in plants and animals. Such environmental issues can be overcome by the use of biologically-essential elements such as Cu(II) and Ni(II) 1-3. Although the pharmaceutical industry has witnessed so many drugs all these years, there is still a need for a most potential drug with minimum side effects. Cancer is a major threat in front of our mankind today and is one of the primary concerns of the field of medicinal inorganic chemistry. When cisplatin was accepted as a chemotherapeutic drug, it revolutionized the therapeutic industry. This paved way for the construction of novel compounds by metal-ligand bonding. Platinum-based complexes had been a research focus for many years, but now there are more explorations in non-platinum based drugs too 4-7. The synthesis of symmetrical and unsymmetrical binuclear Cu(II) and Ni(II) complexes has gained more attention in recent years. Among the variety of methodologies applied to synthesize polynuclear metal complexes, the use of mononuclear complexes as ligands, is very useful and successful 8, 9. Based on this, in the present work, the main focus is the synthesis of homo and hetero binuclear symmetrical and unsymmetrical Cu(II) & Ni(II) complexes with a new Schiff base ligand derived from 4 – chloro-o-phenylenediamine and 3,5-dichloro-2-hydroxyacetophenone.
Experimental Methods
Materials
All chemicals used were of the analytical reagent grade (AR) and of the highest purity available. They included 3,5-dichloro-2-hydroxyacetophenone (sigma) and 4-chloro-o-phenylenediamine (sigma), NiCl2.6H2O (BDH), CuCl2 (sigma). Organic solvents used includes absolute ethyl alcohol, diethylether, chloroform, diethylamine and dimethyl formamide (DMF).
Physical Measurements
UV-Vis spectra of the metal complexes in ethanol were recorded on a Shimadzu UV-3101PC UV-Vis-NIR scanning spectrophotometer between 200 and 800 nm. IR spectra of the ligands and their metal complexes, as KBr discs, were recorded on a FT-IR 1650 Shimadzu spectrometer in the region 4000 – 400 cm−1. 1H NMR spectra of the ligand and its complexes, were recorded in CDCl3 on a 500 MHz Bruker spectrometer at room temperature using TMS as internal standard. Electrochemical experiments were performed with a CHI 760 electrochemical analyzer, in single compartmental cells using tetrabutylammonium perchlorate (TBAP) as a supporting electrolyte. Thermal studies of the synthesized compounds were carried out in the Shimadzu, DTG-60, heating rate 20 degrees/min 20–800 ⁰C range under N2 atmosphere. The EPR spectrum was recorded on an X-band in the solid state using Bruker EMX Plus with Microwave Frequency, 9.865832 GHz at room temperature in DMSO of liquid nitrogen using DPPH free radical. Molar conductivity of 10−3 M solutions of the complexes in ethanol was measured on the ELICO CM 185 conductivity Bridge.
Synthetic Methodology of Schiff Base Ligand and its Mono and Binuclear Complexes
Synthesis of Ligand
The ligand was prepared by adding an ethanolic solution (20 mL) of 3,5-dichloro-2-hydroxyacetophenone (0.4101g; 2mmol) to an ethanolic solution of 4-chloro-o-phenylenediamine (0.1426g; 1mmol) with constant stirring followed by refluxion (3 h). A brown solid was formed on cooling the solution slowly to room temperature and the precipitate was collected by filtration, washed with ethanol, then diethyl ether, and finally air-dried. The yield was 80% 10.
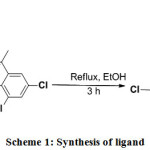 |
Scheme 1: Synthesis of ligand
|
Synthesis Route of Mononuclear Schiff Base Metal Complexes
Mononuclear Cu(II) Complex
The solution of copper(II) acetate mono hydrate (0.1997g; 1mmol) in 20 mL ethanol was added drop wise to a solution of ligand (0.5167g) in ethanol (20 mL) with constant stirring. The mixture was gently heated under reflux for 3 h 11. After refluxing, the resulting solution was kept undisturbed for slow evaporation of the solvent. After four days, the bluish brown colored complex was filtered, washed thoroughly with ethanol and dried in a vacuum.
Mononuclear Ni(II) Complex
Mononuclear Ni(II) complex was prepared by addition of an ethanolic solution (20 mL) of nickel (II) acetate tetrahydrate (0.2488g; 1mmol) to a solution of ligand (0.5167g) in ethanol (20 mL) drop by drop with constant stirring. The mixture was gently heated under reflux for 3 h 11, which resulted in a rapid change of color from brown to greenish brown powder. The greenish brown colored complex was filtered, washed thoroughly with ethanol and dried in a vacuum.
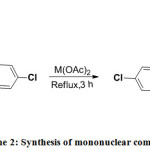 |
Scheme 2: Synthesis of mononuclear complexes
|
Synthetic Route of Homo Binuclear Schiff Base Metal Complexes
Homo Binuclear Cu(II) Complex
The homo binuclear copper(II) complex was synthesized by slow addition of 20 ml ethanolic solution of anhydrous copper (II) chloride (0.1346g; 1mmol) to 20 ml ethanolic solution of mononuclear Cu(II) complex (0.5781g; 1mmol). The resulting mixture was heated under reflux for 3 hrs 12, 13. After refluxing, the resulting solution was filtered and the filtrate was left undisturbed for slow evaporation of the solvent. After five days, the dark blue coloured complex was obtained.
Homo binuclear Ni(II) Complex
The homo binuclear nickel(II) complex was synthesized by slow addition of 20 ml ethanolic solution of Nickel chloride hexahydrate (0.2377g; 1mmol) to 20 ml ethanolic solution of mononuclear Ni(II) complex (0.5634g; 1mmol). The resulting mixture was heated under reflux for 4 hrs 12, 13. After refluxing, the resulting solution was filtered, washed thoroughly with ethanol and dried in a vacuum. After six days, the greenish brown solid complex was obtained.
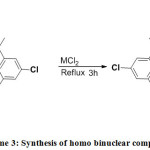 |
Scheme 3: Synthesis of homo binuclear complexes
|
Synthetic Route of Heterobinuclear Schiff Base Metal Complexes
Hetero Binuclear Metal Complex from Mononuclear Cu(II) Complex
The hetero binuclear MM1(II) complex (M = Cu; M1= Ni) was synthesized by slow addition of 20 ml ethanolic solution of nickel chloride hexahydrate (0.2377g; 1mmol) to 20 ml ethanolic solution of mononuclear Cu(II) complex (0.5781g; 1mmol). The resulting mixture was heated under reflux for 3 hrs 12, 13. After refluxing, the resulting solution was filtered, purified with ethanol and dried in a vacuum. After five days, the greenish blue solid complex was obtained.
Hetero Binuclear Metal Complex from Mononuclear Ni(II) Complex
The hetero binuclear M1M(II) complex (M = Cu; M1= Ni) was synthesized by slow addition of 20ml ethanolic solution of anhydrous copper chloride (0.1345g; 1mmol) to 20ml ethanolic solution of mononuclear Ni(II) complex (0.5634g; 1mmol). The resulting mixture was heated under reflux for 3 hrs 12, 13. After refluxing, the resulting solution was filtered and washed with ethanol. The dark green solid product obtained was dried in vacuo.
Pharmacological Applications of Synthesized Compounds
Antibacterial Assay
The synthesized Schiff base and their metal complexes were screened for their antibacterial properties of the disc agar diffusion method in DMF solvent against S. aureus, B. subtilis (Gram-positive bacteria), E. coli and P. bacilli (Gram-negative bacteria). The antibiotic Streptomycin was used as the standard reference in the case. The tested compounds were dissolved in ethanol to get concentration of 10, 20, 30 and 40 mg/L. The test was performed on medium potato dextrose agar contains infusion of 200 g potatoes, 6 g dextrose and 15 g agar. Uniform size filter paper discs (three disks per compound) were impregnated by equal volume from the specific concentration of dissolved tested compounds and carefully placed on incubated agar surface. After incubation for 36 h at 27°C, inhibition of the organism which evidenced by clear zone surrounds each disk was measured and used to calculate mean of inhibition zones14.
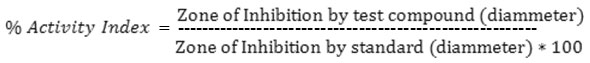
In-Vitro Anticancer Activity
An anticancer activity of the mono, homo and hetero binuclear Schiff base Cu(II) and Ni(II) complexes was performed in the MCF-7 culture medium. Test samples in triplicates were added to the cells. After incubation at 37 °C for 18 hrs, MTT was added in all the wells and incubated for 4 hrs. After incubation, DMSO was added in the wells and read at 570 nm using a photometer. Cytotoxicity and cell viability was calculated by the following formula.
Cytotoxicity= [(Control-Treated)/ control] * 100
Cell viability= (Treated/ Control) * 100.
Results and Discussion
All the metal complexes show molar conductivities in the range of 13.64 – 14.84 ohm-1 cm2 mol-1 (Table 1) in 10-3 M ethanolic solution analogous to non electrolytes. The neutrality of the complexes can be correlated by the deprotonated nature of the ligand with most complexes 15.
Table 1: Analytical data of the Schiff base ligand and its Complexes
| Compounds |
Molecular Formula |
Yield % |
Color |
M.Pt (°C) |
Calculated (Found) % |
Λm (ohm-1cm2mol–1) |
|||||
|
C |
H |
N |
Cl |
Cu |
Ni |
||||||
|
L |
C22H15Cl5N2O2 |
80 |
Brown |
102-105 |
51.15 (50.04) |
2.93 (2.34) |
5.42 (5.21) |
34.31 (34.12) |
– |
– |
– |
|
CuL |
CuC22H13Cl5N2O2 |
70 |
Bluish brown bbrown |
>250 |
45.70 (44.14) |
2.27 (2.17) |
4.85 (4.62) |
30.66 (30.13)
|
10.99 (10.37) |
– |
14.84
|
|
NiL |
NiC22H13Cl5N2O2 |
68 |
Greenish brown |
>250 |
46.09 (45.54) |
2.29 (2.18) |
4.89 (4.77) |
30.92 (30.26) |
– |
10.24 (10.05) |
14.58 |
|
Cu2LCl2 |
Cu2C22H13Cl7N2O2 |
70 |
Dark blue |
>250 |
37.08 (36.78) |
1.84 (1.79) |
3.93 (3.84) |
34.83 (34.08) |
17.83 (17.62) |
–
|
14.52 |
|
Ni2LCl2 |
Ni2C22H13Cl7N2O2 |
67 |
Greenish brown
|
>250 |
37.59 (38.85) |
1.86 (1.81) |
3.99 (3.92) |
35.31 (34.80) |
– |
16.70 (16.56)
|
13.65 |
|
CuNiLCl2 |
CuNiC22H13Cl7N2O2 |
72 |
Greenish blue |
>250 |
37.33 (36.81) |
1.85 (1.82) |
3.96 (3.92) |
35.06 (34.75) |
8.98 (8.86) |
8.29 (8.17) |
14.45 |
|
NiCuLCl2 |
NiCuC22H13Cl7N2O2 |
70 |
Dark green |
>250 |
37.33 (36.64) |
1.82 (1.78) |
3.96 (3.93) |
35.06 (34.72) |
8.98 (8.81) |
8.29 (8.12) |
13.64 |
IR Spectral Studies
IR spectral datas of ligand and its metal complexes make known the involvement of coordination sites in chelation (Table 2). The free ligand showed a characteristic band at 1592 cm-1 and 1438 cm−1 is assigned to azomethine (C=N) and C-O group respectively, which shifted to either higher or lower wavenumber upon coordination in all the complexes supporting the coordination through azomethine nitrogen and phenoxo oxygen atoms 16-20. A sharp vibrational band positioned at 3423 cm-1 is correspond to -OH group belonging to the 3, 5-dichloro-2-hydroxyacetophenone, which disappear in complexes confirms deprotonation of -OH group and coordination of oxygen to the metal. The stronger bands observed in the region 490-545 cm-1 and 550 -754 cm-1 in metal complexes, which has been assigned to M – N and M – O stretching frequencies respectively. IR data confirm the Cu(II) & Ni(II) metal atom binding together with both O and N donor groups of the Schiff base ligand and support the tentative structure of the complexes.
Table 2: Characteristic infrared spectral bands for Schiff base ligand(L) and its Cu(II) & Ni(II) complexes.
| S.No. | Compounds | ν(OH) | ν(C=N) | ν(C-O) | ν(M-O) | ν(M-N) (cm-1) |
| (cm-1) | (cm-1) | (cm-1) | (cm-1) | |||
| 1 | L | 3423 | 1592 | 1438 | – | – |
| 2 | CuL | – | 1505 | 1430 | 754 | 545 |
| 3 | NiL | – | 1610 | 1436 | 550 | 500 |
| 4 | Cu2LCl2 | – | 1610 | 1436 | 563 | 477 |
| 5 | Ni2LCl2 | – | 1643 | 1435 | 667 | 500 |
| 6 | CuNiLCl2 | – | 1629 | 1436 | 750 | 500 |
| 7 | NiCuLCl2 | – | 1618 | 1438 | 748 | 490 |
Electronic Spectral Studies
The electronic spectral characteristics (230–800 nm) of the ligand and complexes in ethanol are given in Table 3. The electronic spectrum of the ligand in ethanol exhibits two bands in the range 220–350 nm due to π→π* and n→π* transitions 21-23. The high intensity band obtained at 220 nm is assigned to intraligand charge transfer transition, π→π* of benzene ring24- 26. The medium energy band at 348 nm was assigned to n→π* transition of the azomethine group 27. The corresponding azomethine band showed a slight shift to longer wavelength on going from ligand to complex, indicating coordination of ligand to metal through the azomethine group. A moderately intensive band observed in the region of 430 – 460 nm is attributable to the ligand → M charge transfer transitions in all the complexes,. The mononuclear Cu(II) complex exhibited a band at 585 nm assigned to 2B1g → 2A1g transition, suggesting square planar geometry around Cu(II) center16. The electronic spectrum of mononuclear Ni(II) complex exhibits two bands at 570 and 630 nm that was assigned to 1A1g →1A2g and 1A1g → 1B1g transition respectively, consistent with square planar geometry28. Homo binuclear Cu(II) complex exhibited one absorption band at 570 nm, which was attributed to 2B1g → 2A1g transition indicating the square planar geometry. Homo binuclear Ni(II) complex exhibits two absorption bands, which fall at 520 and 600 nm attributed to 1A1g →1A2g and 1A1g → 1B1g transitions respectively consistent with that of the square planar geometry. The electronic absorption spectra of the hetero binuclear complexes show absorptions at 560 and 647 nm, which may be recognized to 1A1g →1A2g and 1A1g → 1B1g transitions for Ni(II). The band at 560 nm, which may be an overlapping region with that of the Ni(II) ion, may also be due to 2B1g→2A1g transition of the Cu(II) ion in the hetero binuclear complexes. These values are well within the range reported for square planar complexes.
Table 3: Electronic spectral assignments for Schiff base ligand(L) and its Cu(II) & Ni(II) complexes
| S.No. | Complex | λmax (nm) | Band assignment |
Geometry of the complex |
| 1 | L | 220 | π-π* | – |
| n-π* | ||||
| 348 | ||||
| 2 | CuL | 230 | π-π* | Square planar |
| 362 | n-π* | |||
| 457 | L→M charge transfer | |||
| 585 | 2B1g → 2A1g | |||
| 3 | NiL | 256 | π-π* | Square planar |
| 368 | n-π* | |||
| 436 | L→M charge transfer | |||
| 570 | 1A1g →1A2g, | |||
| 630 | 1A1g → 1B1g | |||
| 4 | Cu2LCl2 | 264 | π-π* | Square planar |
| 380 | n-π* | |||
| 460 | L→M charge transfer | |||
| 570 | 2B1g → 2A1g | |||
| 5 | Ni2LCl2 | 249 | π-π* | Square planar |
| 382 | n-π* | |||
| 430 | L→M charge transfer | |||
| 520 | 1A1g→1A2g | |||
| 600 | 1A1g → 1B1g | |||
| 6 | CuNiLCl2 | 270 | π-π* | Square planar |
| 375 | n-π* | |||
| 460 | L→M charge transfer | |||
| 560 | 1A1g→1A2g | |||
| 647 | 1A1g→1B1g | |||
| 7 | NiCuLCl2 | 262 | π-π* | Square planar |
| 347 | n-π* | |||
| 437 | L→M / CT | |||
| 534 | 1A1g →1A2g | |||
| 615 | 1A1g→B1g |
1H NMR Spectral Studies
To elucidate the structure and nature of ligand, the 1HNMR spectra of the Schiff base ligand was recorded in CDCl3 using TMS as the reference compound at room temperature (fig. 1). The multiplet at δ= 6.6 – 7.6 ppm correspond to aromatic protons 29. The singlet peak appears at δ = 12.738 ppm is attributed to the phenolic -OH group present and additional peak appear in the range of δ = 2.402 ppm is attributed to the methyl protons of the ligand 30.
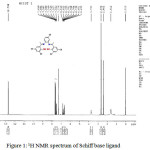 |
Figure 1: 1H NMR spectrum of Schiff base ligand
|
Cyclic voltammetry
The electro chemical behavior of metal complexes was studied by cyclic voltammetry in DMSO containing TBAP as supporting electrolyte between the range of 2.0 to -2.0 V. The electrochemical data such as cathodic peak potential (Epc), anodic peak potential (Epa), peak separation (ΔEp) and redox potential, E1/2 are given in Table 4. The mononuclear Cu(II) and Ni(II) complexes shows cathodic peak potential at, Epc = -0.4 and -0.5 V, with an associated anodic peak at, Epa = -0.2 and – 0.1 V, ΔEp= 200 and 400 and E1/2 = -0.35 and -0.3. Since ΔEp value indicates a quasireversible one electron transfer redox behavior mononuclear Cu(II) and Ni(II) complexes 31.
Cu(II) ↔ Cu(I)
Ni(II) ↔ Ni(I)
The ΔEp and E1/2 values shows that all the homo and hetero binuclear Cu(II) & Ni(II) complexes exhibit two one electron anodic and cathodic responses 32 and the processes are expressed as follows:
CuII CuII ↔ CuII CuI ↔ CuI CuI
NiII NiII ↔ NiII NiI ↔ NiI NiI
CuII NiII ↔ CuII NiI ↔ CuI NiI
Thermal Analyses (TG/DTA)
To determine the thermal stability and chemical composition of Schiff base metal complexes, TG and DT analysis has been carried out in a temperature range of 20–800°C/min and was shown in Figure 2(a-d). The thermal behaviour of all the Schiff base Cu(II) & Ni(II) complexes was almost similar. Mononuclear Cu(II) complex decomposed into two stages. The first stage, at 130 – 270°C/min, corresponds to the loss of five chloride ions (exp.30.45% ; calcd. 30.66 %). The process of elimination is also confirmed by DTA curve at 220°C. In the second stage, it loses C22H13N2O (exp. 56.56, calcd. 55.52 %) with formation of metal oxide (CuO) 33, 34 at 360 – 780°C/min and is further conformed by DTA curve at 640°C. Ni(II) complex degraded in two steps. The first step resulted in mass loss of 30.75 % (calcd. 30.92 %) corresponding to the loss of five chloride ions within the temperature range 130 – 190°C. This process of elimination is also indicated by 157°C on DTA curve. The second step involved weight loss of 55.27 % (calcd. 55.99 %) corresponding to the removal of the remaining part of the ligand (temperature range 305- 750°C) leaving metal oxide (NiO) with DTA curve at 630°C. The overall weight losses for the complexes are in good agreement with the proposed formula obtained by elemental analyses, IR and UV data’s. In the same way decomposition of homo and hetro binuclear complexes are given the Table 4. From the results, it is well evident that the hetero binuclear complex decomposes at a higher temperature than compared to mononuclear and homo binuclear complexes.
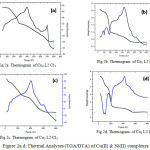 |
Figure 2a.d: Thermal Analyses (TGA/DTA) of Cu(II) & Ni(II) complexes Click here to View figure |
 
Table 4: Thermal analysis data of Schiff base ligand(L) and its metal(II) complexes
|
Complexes |
Temperature range of decomposition(ºC) |
% of weight loss |
Assignment |
|
CuL |
130-270 |
30.45(30.66) |
Loss of 5 Cl |
| 360-780 |
56.56(55.52) |
Loss of C22H13N2O with the formation of CuO |
|
| NiL
|
130-190 |
30.75(30.92) |
Loss of 5 Cl |
|
305-750 |
55.27(55.99) |
Loss of C22H13N2O with the formation of NiO | |
|
Cu2LCl2 |
120-260 |
31.80(34.38) |
Loss of 7 chloride ions |
|
410-790 |
40.36(42.80) |
Loss of C22H13N2 with the formation of 2CuO | |
|
Ni2LCl2 |
145-215 |
49.46(50.93) |
Loss of C12H4Cl6 |
|
430-790 |
23.71(27.32) |
Loss of C10H9N2Cl with formation of NiO
|
|
| CuNiLCl2
|
130-260 |
24.67(27.01) |
Loss of 6 chloride ions |
|
370-760 |
46.12(48.04) |
C22H13N2Cl with the formation of CuO and NiO
|
|
| NiCuLCl2 |
140-200 |
34.37(34.61) |
Loss of 7chloride ions |
|
460-780
|
41.08(43.09)
|
Loss of C22H13N2 with formation of NiO and CuO |
EPR Spectra
The X-band EPR spectra of mononuclear, homo and hetero-binuclear Cu(II) complexes were recorded at room temperature using DPPH as a reference (Figure 3(a-d)). The corresponding data are given in the Table 5. The g values observed for mononuclear Cu(II) complex g‖ = 2.2342, g┴ = 2.1026, G = 2.28. The g‖ and g┴ values are greater than 2.04 which confirmed the existence of unpaired electron in the dx2-y2 orbital corresponds to square planar geometry around the Cu(II) center35 is an important function for indicating covalent character of M-L bonds 36. For ionic character, g‖ > 2.30 while in covalent character g‖ < 2.30. In the present compounds, the g‖ < 2.30 indicating appreciable covalent character for M-L bond.The exchange interaction between copper centers are measured by the expression
G = (g‖ – 2)/(g┴ – 2).
The calculated G values for these complexes are in the range 2.28–3.9315 which predicts the weak exchange interaction.
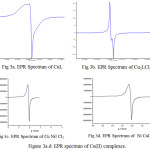 |
Figure 3a.d: EPR spectrum of Cu(II) complexes. |
Table 5: EPR spectral parameters of Schiff base Cu(II) & Ni(II) complexes
|
S.No |
Complex |
g‖ |
g┴ |
Δg |
G |
|
1. |
CuL |
2.2342 |
2.1026 |
0.1316 |
2.28 |
|
2. |
Cu2LCl2 |
2.2301 |
2.1026 |
0.1274 |
2.2417 |
|
3. |
CuNiLCl2 |
2.1781 |
2.0453 |
0.1328 |
3.9315 |
|
4. |
NiCuLCl2 |
2.1781 |
2.0526 |
0.1255 |
3.3859 |
Antibacterial Screening of Schiff Base And Its Complexes
In recent times pharmaceutical industries are looking for synthesizing the alternative compounds which act as a drug. At present much consideration has been focussed on the synthesis of new metal complexes and the evaluation of these agents for antibacterial activity37. S. aureus, B. substils (Gram-positive) and E. coli, P.bacilli (Gram-negative) are the general bacterias that are found in the contaminated wound. The synthesized homo and hetero binuclear complexes were tested against Gram positive and Gram negative stains at different concentrations like 10, 20, 30 and 40 (μg/mL) which are compared with control (Streptomycin) (Figure 4). From the figure, it is well evident that homo binuclear Schiff base copper(II) complexes have higher antibacterial activity against both Gram-positive and Gram-negative bacteria stains. The variation in the effectiveness of different compounds against different organisms depends either on the impermeability of the cells of the microbes or on divergence in ribosome of microbial cells. Current studies reveal that the metal ions with high atomic radius and electronegativity in their metal complexes exhibit high antimicrobial activity. In particular the complexes showed excellent activity against E. coli which is due to the differences in the cell wall structure. The cell wall of the gram-positive bacteria are made of a thick layer of peptidoglycan, consisting of linear polysaccharide chains leading to difficult penetration compared to the gram-negative bacteria where the cell wall possesses thinner layer of peptidoglycan. The increased antibacterial activity of the metal complexes suggests that the chelation could facilitate the ability of a complex to cross a cell membrane and can be explained by Tweedy’s chelation theory 38. Chelation considerably reduces the polarity of the metal ion because of partial sharing of its positive charge with donor groups and possible electron delocalization over the whole chelate ring. Such a chelation could enhance the lipophilic character of the central metal atom, which subsequently favors its permeation through the lipid layer of the cell membrane.
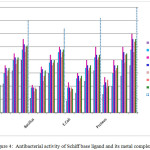 |
Figure 4: Antibacterial activity of Schiff base ligand and its metal complexes
|
In-Vitro Cytotoxic Activity
The anticancer activity of the synthesized metal complexes was studied by treating it with MCF-7cell line (human breast cancer cell) and read at 570 nm using a photometer by applying MTT assay. The results were analyzed by cell viability curves, expressed with IC50 values in the studied concentration range of 2.5 – 100 μg/mL (Figure 5). The MTT cell proliferation assay has been widely accepted as a reliable way to measure the cell propagation rate 39. The result showed that the complexes inhibited the growth of MCF-7 cells in a dose-dependent manner. In comparision the highest level of cytotoxic activity was observed in homo-binuclear Cu(II) complex (IC50= 7.3789). The results indicate that these complexes have beneficial features for potential anticancer agents.
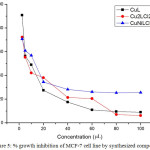 |
Figure 5: % growth inhibition of MCF-7 cell line by synthesized compounds Click here to View figure |
Conclusion
Schiff base and its mononuclear, homo and hetero-binuclear metal complexes have been synthesized successfully and characterized by various physico chemical methods such as molar conductance, FT-IR, UV-Vis,1HNMR, cyclic voltammetry, thermal analysis and EPR. All the metal complexes are square planar environment which was confirmed by electronic and EPR spectral data. The cyclic voltammetry result supported that the mononuclear complexes exhibit one step one electron transfer, whereas binuclear metal complexes exhibit two steps one electron transfer and quasi-reversible nature. From thermal analysis, it was concluded that binuclear metal complexes were more stable than mononuclear metal complexes. From the result of antibacterial activity test, we conclude that the hetero-binuclear Cu(II) complex plays an effective role in improving the bioactivity. The anticancer activity result shows that homo binuclear Cu(II) complex has high cytotoxicity.
References
- Rosenberg, B.; VanCamp, L.; Trosko, J. E.; Mansour, V. H.; Nature, 1969, 222(5191), 385–386.
CrossRef - Jungwirth, U.; Kowol, C. R.; Keppler, B. K.; Hartinger, C. G.; Berger, W.; Heffeter, P.; Liebert, M. A.; Inc., publishers, 2011, 15(4), 1085-127.doi:10.1089/ars.2010.3663.
CrossRef - Sinn, E.; Harris, C. M.; Coord. Chem. Rev., 1969, 4, 4391−4422.
CrossRef - Kuby, S. A.; Mechanism of Enzyme Action; CRC Press: Boca Raton, FL, 1990.
- Trudu, F.; Amato, F.; Vaňhara, P.; Pivetta, T.; Peña-Méndez, E. M.; Havel, J.; J. App. Biomed., 2015, 13, 79-103.
CrossRef - Tisato, F.; Marzano, C.; Porchia, M.; Pellei, M.; Santini, C.; Med. Res. Rev., 2010, 30, 708-749.
- Kim, A.Y.; Seok, W.K.; Dong, Y.; Yun, H.; Inorg. Chim. Acta, 2001,319, 194.
CrossRef - Ramachandraiah, A.; Sarojini, T.; Laxma reddy, K.; Polyhedron, 1990, 9, 1703-1708.
CrossRef - Zacharias, P. S.; Ramachandraiah, A.; Polyhedron, 1985, 4, 1013-1017.
CrossRef - Hamil, A. M.; Abdelkarem, M.; Hemmet, M.; El-ajaily, M. M.; J. ChemTech Res., 2012, 4, 682-685.
- Al-Shaalan, N. H.; Molecules, 2011, 16, 8629-8645.
CrossRef - Vidal, M.; Rizzardi, G.; Vigato, P. A.; Casellato, U.; Kida, S.; Okawa, H.; Inorg. Chimica Acta, 1979, 34, 19-24.
CrossRef - Fenton, D. E.; Gayda, S. E.; Casellato, U.; Vidali, M.; Vigato, P. A.; Inorg. Chim. Acta, 1978, 27, 9-14.
CrossRef - Chohan, Z. H.; Supuran, C. T.; Scozzafava, A.; J. Enzyme Inhib. Med. Chem., 2004, 19(1), 79-84.
CrossRef - Geeta, B.; Shravankumar, K.; Muralidhar Reddy, P.; Ravikrishna, E.; Sarangapani, M.; Krishna Reddy, K.; Ravinder, V.; Spectrochim. Acta Part A, 2010, 77, 911-915.
CrossRef - Sharma, R. K.; Sing, R.V.; Tandon, J. P.; J. Inorg. Nucl. Chem, 1980, 42, 1382-1384.
CrossRef - Raman, N.; Sobha, S.; Spectrochim. Acta Part A, 2012, 85, 223-234.
CrossRef - Zhang, J.; Braunstein, P.; Welter, R.; Inorg. Chem, 2004, 43, 4172-4177.
CrossRef - Dutta, B.; Bag, P.; Adhikary, B.; Florke, U.; Nag, K.; J. Org. Chem., 2004, 69(16), 5419-5427.
CrossRef - Pattanayak, P.; Pratihar, J.L.; Patra, D.; Brandão, P.; Mal, D.; Felix, V.; Polyhedron, 2013, 59, 23-28.
CrossRef - Pattanayak, P.; Pratihar, J.L.; Patra, D.; Her Lin, C.; Brandão, P.; Mal, D.; Felix, V.; J. Coord. Chem., 2013, 66, 568-579.
CrossRef - Anbu, S.; Kamalraj, S.; Varghese, B.; Muthumary, J.; Kandaswamy, M.; Inorg. Chem., 2012, 51, 5580-5592.
CrossRef - Fekri, R.; Shaabani, B.; J. Appl. Environ. Biol. Sci., 2013, 3, 75-78.
- MacLachlan, M. J.; Park, M. K.; Thompson, L. K.; Inorg. Chem, 1996, 35, 5492-5499.
CrossRef - Nagakavitha, D.; Hussainreddy, K.; Int J Pharm Bio Sci., 2014, 5(4), 294-304.
- Tas, E.; Kilic, A.; Konak, N.;, Yilmaz, I.; Polyhedron, 2008, 27, 1024-1032.
CrossRef - Rosenthal, M. F.; J. Chem. Educ., 1973, 50, 331-335.
CrossRef - Thomas, M.; Nair, M.K.M.; Radhakrishnan, R.K.; Synth.React.Inorg. Met.Org, 1995, 25, 471-479.
CrossRef - Silverstein, R. M.; Bassler, C. G.; Morril, T. C.; Spectrophotometric Identification of Organic Compound, 5th edn., New York, Wiley, 1991.
- Breitmaier, E.; Structure Elucidation by NMR in Organic Chemistry, United Kingdom, John Wiley & Sons, 2002.
- Raman, N.; Ravichandran, S.; Thangaraja, C.; J. Chem. Sci., 2004, 116, 215-219.
CrossRef - Anupama, B.; Padmaja, M.; GyanaKumari, C.;. E-Journal of Chemistry, 2012; 9(1), 389-400.
CrossRef - Nakamoto, K.; Infrared and Raman spectra of inorganic and coordination compounds, wiley, 3rd edn., New York, 1997.
- El- Boraey, H. A.; Ayad, M. I.; Int.J. ChemTech Res, 2014, 6(1), 266-275.
- Prabhumirashi, L. S.; Khoje, J. K.; Indian J Chem, 2004, 43A, 299-302.
- Shakir, M.; Abbasi, A.; Khan, A. U.; Khan, S. .N.; Spectrochim. Acta Part A, 2011, 78, 29-35.
CrossRef - Wei, Q. Y.; Xiong, J. J.; Jiang, H.; Zhang, C.; Ye, W.; Int. J. Food Microbio., 2011, 150, 164-170.
CrossRef - Tweedy, B. G.; Phytopathology, 1964, 55, 910-914.
- Marques, M. P. M.; Gira, T.; Lima, M. C. P. D.; Gameiro, A.; Pereira, E.; Garcia, P.; Biochim. Biophys. Acta, 2002, 1589, 63-70.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









