A Novel and Green Method for N-acylation of Amines and Sulfonamides under Ultrasound Irradiation
Radia Bouasla1,2, Khaoula Bechlem1, Billel Belhani1, Ismahene Grib1, Nour-Eddine Aouf1 and Malika Berredjem1
1Laboratory of Applied Organic Chemistry, Synthesis of biomolecules and molecular modelling Group, Department of chemistry, Badji-Mokhtar - Annaba University, Box 12, 23000 Annaba, Algeria.
2Higher School of Industrial Technologies-Annaba, Box 218, 23000 Annaba, Algeria.
Corresponding Author E-mail: malika.berredjem@univ-annaba.org
DOI : http://dx.doi.org/10.13005/ojc/330348
A facile and versatile method for acylation of structurally diverse amines and sulfonamides under focused ultrasonic irradiation in catalyst-free and solvent-free conditions is reported. There are several advantages to this approach such as simple and easier workup conditions, small amount of time and high yielding. The acylation reaction was carried out with acetic anhydride. All structures of synthesized products have been identified by NMR and mass spectroscopy.
KEYWORDS:N-acylation; sulfonamide; ultrasonic irradiation; acetic anhydride; green chemistry
Download this article as:| Copy the following to cite this article: Bouasla R, Bechlem K, Belhani B, Grib I, Aouf N, Berredjem M. A Novel and Green Method for N-acylation of Amines and Sulfonamides under Ultrasound Irradiation. Orient J Chem 2017;33(3). |
| Copy the following to cite this URL: Bouasla R, Bechlem K, Belhani B, Grib I, Aouf N, Berredjem M. A Novel and Green Method for N-acylation of Amines and Sulfonamides under Ultrasound Irradiation. Orient J Chem 2017;33(3). Available from: http://www.orientjchem.org/?p=33405 |
Introduction
The N-acylsulfonamide moiety is a common feature in several important biomolecules and has emerged as an important class drug synthesis1-3. A large number of novel N-acylated-sulfonamides molecules have been investigated for antibacterial activity4. They are also known as therapeutic agents for Alzheimer’s disease5and anti-proliferative agents6.
In the literature, several reaction pathways for the preparation of N-acylsulfonamides have been developed, most of them were carried out by the condensation of the various sulfonamides with acyl chloride or anhydride under basic conditions7. Acylation of sulfonamides can also be achieved with concentrated H2SO48, Lewis acids9or heterogeneous solid acid10in acetonitrile under acidic conditions. Another attractive method based on using of palladium-catalyzed acylation of aryl with sulfonamides under microwave irradiation11.
Recently, an efficient method for acylation of sulfonamides in acetonitrile with H6P2W18O62 as acidic solid catalyst is described by Bougheloum et al12. Synthetic methods should be developed, to produce molecules that are no toxic to human health and the environment, because we are all inhabitants on Earth, everyone is a stakeholder, and every person aims contributing to environmental protection locally, nation wide and worldwide.
Chemists have focused on maximizing yield, reducing energy and minimizing the number of steps by application of the green chemistry principle of atom economy to chemical reactions.
Chemical synthesis usually requires an energy source; there are several types of traditional energy such as: light, heat and ionizing radiation. Sonication chemistry can be replaced the traditional energy used for the chemical reaction by irradiation ultrasonic.
In this protocol, ultrasound provides strange mechanism for supplying high energy chemistry, based on acoustic cavitation 13-16. It was suggested that sonication also assists in the breakdown of intermediates and desorption of the products from the surface, The use of ultrasound in chemical reactions provides specific activation based on a physical-chemical phenomena which are currently a matter of advanced research17-18. In recent years, ultrasound irradiation has been considered as a successful method in organic synthesis, because it has several advantages such as; shorter reaction time, higher yield and milder conditions19-22.
Herein, we describe a practical procedure for the acylation of various amines and sulfonamides with acetic anhydride at room temperature and without a solvent using ultrasonic irradiation.
Experimental
General
The sulfonamide derivatives were prepared according to the literature23. All reactions were monitored by thin layer chromatography using Merck TLC Silica gel 60 F254, using the solvents indicated. Silica gel column chromatography was performed over. Sonication was performed in a FUNGILAB ultrasonic bath at a frequency of 40 kHz and nominal power 260 W. Melting points were measured in open capillary tubes on an electro thermal-apparatus.
1H-NMR and 13C-NMR spectra were recorded on Bruker spectrometer (400 and 100MHz) using (CDCl3) as solvent and (TMS) as an internal standard. IR spectra were recorded on a Perkin- Elmer FT-600 spectrophotometer. Mass spectra were performed on a Shimadzu QP operating at an IP of 30 or 70 eV.
Typical Experimental Procedure for N-Acylation of Amines and Sulfonamides
In a glass tube was placed a mixture of amine or sulfonamide derivatives (1 equiv) and acetic anhydride (1equiv) at room temperature. The mixture was immersed in the water of the
sonicator bath for appropriate time at room temperature. Reaction was monitored by TLC.
After completion of the reaction, the reaction mixture was diluted with the water, and extracted with ethyl acetate, the organic layer was dried over anhydrous sodium sulfate and the resulting residue was purified by silica gel chromatography using dichloromethane-petroleum ether (9:1), to give the N-acylamines or N-acylsulfonamides in good yieds.
N-Phenylacetamide
Oil; 88 % yield; 1H NMR (CDCl3): 7.2-7.5 (m, 5H, Ar), 2.2 (s, 3H, CH3); 13C NMR (CDCl3): 168.9, 138.5, 128.9, 128.0, 121.6, 24.0; LCMS (+) m/z, 136.07 [M+H]+, C8H9NO.
N-(4-Methoxyphenyl)acetamide
Oil; 89 % yield; 1H NMR (CDCl3): 6.9-7.4 (m, 4H, Ar), 2.2 (s, 3H, CH3), 3.6 (s, 3H, CH3); 13C NMR (CDCl3): 168.9, 158.9, 130.8, 122.6, 114.5, 55.8, 24.2; LCMS (+) m/z, 166 [M+H]+, C9H11NO2.
N-(3,4-Dihydroisoquinolin-2(1H)-yl)acetamide
Powder; 70 % yield; mp 110-111°C; IR (KBr): 1660 (CO); 1H NMR (CDCl3): 7.2–7.0 (m, 4H, Ar), 4.4 (s, 2H, CH2), 3.4 (t, 2H, J5.5 Hz, CH2), 2.6 (t, 2H, J5.8 Hz, CH2), 2.1 (s, 3H, CH3); 13C NMR (CDCl3): 169.2, 134.1, 127.7, 127.5, 126.9, 126.3, 49, 47, 28.6, 21.1; LCMS (+) m/z, 176.10 [M+H]+, C11H13NO.
N-(4-Phenylpiperazin-1-yl)acetamide
Powder; 74 % yield; mp 113-114°C; IR (KBr): 1752 (CO);1H NMR (CDCl3): 7.45-6.95 (m, 5H, Ar), 3.62 (t, 4H, J5.2, 4.8 Hz, CH2), 3.25 (t, 4H, J5.2, 4.8 Hz, CH2), 2.1 (s, 3H, CH3); 13C NMR (CDCl3):168.9, 149.6, 129.6, 121.9, 114.3, 53.3, 46.2, 21.1; LCMS (+) m/z, 205.13 [M+H]+, C12H16N2O.
N-(N-Cyclohexylsulfonyl)acetamide
Powder; 94 % yield; mp 198-199°C; IR (KBr): 3372 and 3265 (NH), 1730(CO), 1360 and 1153 (SO2); 1H NMR (CDCl3): 1.6-2.1 (m, 10H, CH2), 5.2 (d, 1H, J7.35 Hz, CH), 2.0 (s, 3H, CH3);13C NMR (CDCl3): 171.0, 43.9, 32.6, 25.7, 24.8, 21.6; LCMS (+) m/z, 222.09 [M+H]+, C8H16N2O3.
N-(N-Propylsulfonyl)acetamide
Powder; 90 % yield; mp 168-169°C; IR (KBr): 3370 and 3263(NH), 1721(CO), 1380, 1154(SO2); 1H NMR (CDCl3): 3.2 (m, 2H, CH2), 2.1 (s, 3H, CH3), 1.65 (m, 2H,CH2), 1.01 (t, 3H, J 5.02 Hz, CH3);13C NMR (CDCl3): 172.1, 42.5, 21.9, 21.6, 11.2; LCMS (+) m/z, 181.06 [M+H]+,C5H12N2O3S.
N-(N-(Tert-butyl)sulfamoyl)acetamide
Powder; 95 % yield; mp 136-137°C; IR (KBr): 3374 and 3267 (NH), 1735 (CO), 1362 and 1154 (SO2); 1H NMR (CDCl3): 1.15 (s, 9H, 3CH3), 2.01 (s, 3H, CH3); 13C NMR (DMSO-d6): 19.1, 30.6, 30.6, 30.6, 38.7; MS (+) m/z, 212.07 [M+NH4]+, C6H14N2O3S.
N-(N-Benzylsulfamoyl)acetamide
Powder; 96% yield; mp 121-122°C; IR (KBr): 3371 and 3268 (NH), 1738 (CO), 1363 and 1156 (SO2); 1H NMR (CDCl3): 1.90 (s, 3H, CH3), 4.06 (s, 2 H, CH2), 7.2 (m, 1H, Ar); 13C NMR (DMSO-d6): 22.9, 44.2, 125.8, 126.2, 126.7, 127.9, 128.6, 142.3; MS (+) m/z, 246.06 [M+NH4]+, C9H12N2O3S.
N-(N-(3-Fluorophenyl)sulfamoyl)acetamide
Powder; 93 % yield; mp 147-148°C; IR (KBr): 3260 and 3068 (NH), 1694 (CO), 1368 and 1162 (SO2); 1H NMR (CDCl3): 1.95 (s, 3 H, CH3), 6.9 (m, 1H, Ar), 7.06 (m, 2H, Ar), 7.3 (m, 1H, Ar); 13C NMR (DMSO-d6): 22.9, 105.6, 110.1, 114.6, 130.7, 139.5, 163.9, 168.6; MS (+) m/z, 250.04 [M+NH4]+, C8H9N2O3SF.
N-(3,4-Dihydroisoquinolin-2(1H)-yl-sulfonyl)acetamide
Cristal; 90% yield; mp 173-174 °C; IR (KBr): 3261 (NH), 1695 (CO), 1361 and 1155 (SO2); 1H NMR (CDCl3): 8.1 (s, 1H, NH), 7.2-7.0 (m, 4H, Ar), 4.5 (s, 2H, CH2), 3.7 (t, 2H, J 5.7 Hz, CH2), 2.9 (t, 2H, J5.8 Hz, CH2), 2.1 (s, 3H, CH3); 13C NMR (CDCl3): 173, 134.1, 127.5, 126.9, 126.3, 125.7, 48.1, 47.2, 25.3, 21.6; MS (+) m/z, 255.07 [M+NH4]+, C11H14N2O3S.
N-((4-Phenylpiperazin-1-yl)sulfonyl)acetamide
Cristal; 94 % yield; mp 176-177 °C; IR (KBr): 3260 (NH), 1697(CO), 1361 and 1155 (SO2); 1H NMR (CDCl3): 7.7-6.9 (m, 5H, Ar), 3.1 (t, 4H, J5.4 Hz, CH2), 2.5 (t, 4H, J5.2 Hz, CH2), 2.3 (s, 3H, CH3); 13C NMR (CDCl3): 173, 149.6, 129.6, 121.9, 114.3, 50.3, 43.4, 21.5; LCMS (+) m/z, 284.10 [M+H]+, C12H17N3O3S.
N-(N-(4-Methoxyphenyl)sulfamoyl)acetamide
Powder; 80% yield; mp 151-152°C; IR (KBr): 3260 and 3251 (NH), 1690 (CO), 1365 and 1158 (SO2); 1H NMR (CDCl3): 7.1-7.5 (m, 4H, Ar), 3.8 (s, 3H, CH3) 2.3 (s, 3H, CH3); 13C NMR (CDCl3): 171, 153.3, 130, 124.5, 115.1, 55.8, 21.6; LCMS (+) m/z, 245.05 [M+H]+, C9H12N2O4.
(R)-N-(N-(1-Phenylethyl)sulfamoyl)acetamide
Powder; 85 % yield; mp 171-172°C; IR (KBr): 3370 and 3265 (NH), 1698 (CO), 1361and 1152 (SO2); 1H NMR (CDCl3): 8.1 (s, 1H, NH), 7.5-7.2 (m, 5H, Ar), 5.9 (s, 1H, NH), 5.15 (m, 1H, *CH), 2.00 (s, 3H, CH3), 1.50 (d, 3H, J 6.9 Hz, CH3);13C NMR (CDCl3): 171.3; 145.1; 128.2; 126.8; 126.4; 47.2; 21.0; 20.6 ppm; LCMS (+) m/z, 243.07 [M+H]+, C10H14N2O3S.
Ethyl-1-(N-acetylsulfamoyl)pyrrolidine-2-carboxylate
Powder; 83 % yield; mp 199-200°C; IR (KBr): 3254 (NH), 1733 (CO), 1692 (CO), 1369 and 1166 (SO2); 1H NMR (CDCl3): 8.7 (s, 1H, NH), 4.7 (m, 1H, *CH), 4.3 (q, 2 H, J 2.4 Hz, CH2), 3.4-3.7 (m, 6H, CH2), 2.1 (s, 3H, CH3), 1.3 (t, 3H, J 2.4 Hz, 3H, CH3);13C NMR (CDCl3): 171.1, 171.0, 61.3, 60.6, 55.2, 27.7, 21.4, 21.6, 14.1; LCMS (+) m/z, 265.08 [M+H]+, C9H16N2O5S.
Results and Discussion
Following our efforts to develop novel methods for acylation24-27, 12and in continuation of our investigation on the use of ultrasound irradiation to accelerate reactions28-30, we describe a novel method for acylation the different amines and sulfonamides with acetic anhydride under ultrasonic irradiation, in absence of solvent and catalyst.
The main mechanism of sonochemical was the formation of cavitation bubbles 31, under certain conditions, some bubbles implode, generating very high temperatures and pressures, these transient, localized hot spots drive high-energy chemical reactions32-34.
In order to carry out the acylation of the amine function under ultrasonic irradiation, the amine was chosen as a model substrate, the reaction of amine with acetic anhydride using ultrasonic irradiation as shown in scheme 1, the reaction was completed at 15-30 min, the acylated products were isolated in good yields (Scheme 1).
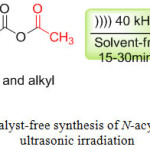 |
Scheme 1: Catalyst-free synthesis of N-acylamines under ultrasonic irradiation. Click here to View scheme |
The N-acylation of amines was successfully achieved using acetic anhydride under ultrasound irradiation. The reactions proceed smoothly in high yields (70-89 %) and in short reaction times. The structures of all compounds were unambiguously confirmed by spectroscopic methods. Proton nuclear magnetic resonance spectra showed singlets at 2.20-2.25 ppm corresponding to the proton of CH3 acetyl. Infrared spectra showed bands at 1650-1700 cm-1 of functional group C=O.
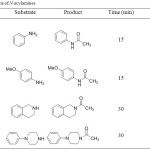 |
Table 1: Synthesis of N-acylamines.
|
Encouraged by this result, we extended the reaction to sulfonamides derivatives using ultrasonic irradiation (Scheme 2). The results are reported in (Table 2, entry 5-14). In all cases, we obtained the corresponding products N-acylsulfonamides in good to excellent yields.
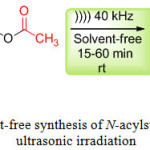 |
Scheme 2: Catalyst-free synthesis of N-acylsulfonamides under ultrasonic irradiation.
|
For the resulting products, Infrared spectra showed band at 1650-1700 cm-1 of functional group (C=O) of acetyl moiety and band at 1170-1395 cm-1 of sulfonyl group. In Proton nuclear magnetic resonance spectra, the structure of N-acylsulfonamides was confirmed by a singlet at 2.27 ppm corresponding to CH3 group.
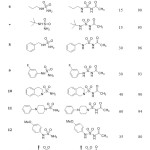 |
Table 2: Synthesis of N-acylsulfonamides. |
The structures of all molecules synthesized were confirmed by usual spectroscopic methods, IR, 1H NMR, 13C NMR and mass spectrometry.
Conclusion
In summary, the acylation of amines or sulfonamides was successfully achieved using acetic anhydride under ultrasound irradiation. This methodology provides a green and much improved approach over the classic methods. Ultrasound can be an effective technology for process intensification in with cavitational collapse can affect chemical transformations. The use of ultrasound is expected to enhance the rate of reaction by various mechanisms.
The non-conventional method offer advantages over conventional process, short time span to complete reaction, easy work procedure and excellent yields.
Acknowledgements
We gratefully acknowledge the support of this work by DG-RSDT and Algerian Ministry of Scientific Research.
References
- Scozzafava, A.; Owa, T.; Mastrolorenzo, A.; Supuran, C. T.Curr. Med. Chem. 2003,10, 925-953.
CrossRef - Rathish, I. G.; Kalim, J.; Shamim, A.; Sameena, B.; Alam, M. S.; Mymoona, A.Eur. J. Med. Chem. 2012, 49, 304-309.
CrossRef - Yildirim, A.; Atmaca, U.; Keskin, A.; Topa, M.; Celik, M.; Gulcin, I.Bioorg. Med. Chem. 2015, 23, 2598-2605.
CrossRef - Banwell, M. G.; Crasto, C. F.; Easton, C. J.; Forrest, A. K.; Karoli, T.; March, D. R.; Mensah, L.; Nairn, M. R.; O’Hanlon, P. J.; Oldham, M. D.; Yue, W.Bioorg. Med. Chem. Lett. 2000, 10, 2263-2267.
CrossRef - Hasegawa, T.; Yamamoto, H. Bull. Chem. Soc. Jpn. 2000, 73, 423-428.
CrossRef - McCuskey, A.; Keane, M. A.; Mudgee, L. M.; Sim, A. T.; Sakoff, J.; Quinn, R.Eur. J. Med. Chem. 2000, 35, 957-964.
CrossRef - Drummond, J.T.; Johnson, G.; Tetrahedron Lett. 1988, 29, 1653-1656.;Guzzo, P. R.; Teng, M.; Miller, M. J.Tetrahedron. 1994, 50, 8275-8292.
- Massah.; A. R.; Asad, B.; Hoseinpour, M.; Molseghi, A.; Kalbasi, R. J.; Naghash, H. J. Tetrahedron. 2009, 65, 7696-7705.
- Raji Reddy, C.; Mahipal, B.; Yaragorla, S. R. Tetrahedron Lett. 2007, 48, 7528-7532.
CrossRef - Singh, D. U.; Singh, P. R.; Samant, S. D. Tetrahedron Lett. 2004, 45, 4805-4982; Massah, A.; Reza, Momeni, A.; Reza, Dabagh.; M.; Aliyan, H.Synth. Commun.2008, 38, 265-273.
- Wu, X.; Rönn, R.; Gossas, T.; Larhed, M.J. Org. Chem. 2005, 70, 3094-3098.
CrossRef - Bougheloum, C.; Barbey, C.; Berredjem, M.; Messalhi, A.; Dupont, N.J. Mol. Struct. 2013, 1041, 6-15.
CrossRef - Safari, J.; Zarnegar, Z.Ultrason. Sonochem.2014, 21, 1132-1139.
CrossRef - Banitaba, S. H.; Safar, J.; Dkhalili, S.Ultrason. Sonochem. 2013, 20, 401-407.
CrossRef - Dilbeck, G. A.; Field, L.; Gallo, A. A.; Gargiulo, R. J.J. Org. Chem. 1978, 43, 4593-4596.
CrossRef - Khaligh, N. G.; Shirin, F.Ultrason. Sonochem.2013, 20, 26-31.
CrossRef - Cravotto, G.; Cintas, P.Chem. Soc. Rev. 2006, 35, 180-196.
CrossRef - Gholap, R.; Venkatesan, K.; Daniel, T.; Lahoti, R. J.; Srinivasan, K. V.Green Chem. 2004, 6, 147-150.
CrossRef - Driowya, M.; Puissant, A.; Robert, G.; Auberger, P. ; Benhida, R. ; Bougrin, K.Ultrason. Sonochem. 2012, 19, 1132-1138.
CrossRef - Belhani, B.; Bouzina, A.; Aouf, N. E.; Berredjem, M.Monatsh. Chem. 2015, 146, 1871-1875. Belhani, B.; Berredjem, M.; Le Borgne, M.; Bouaziz, Z.; Lebreton, J.; Aouf, N. E. RSC Adv. 2015, 5, 39324-39329.
CrossRef - Nasrollahzadeh, M.; Ehsani, A.; Rostami-Vartouni, A. Ultrason. Sonochem. 2014, 21, 275-282.
CrossRef - Bouzina, A.; Berredjem, M.; Bouacida, S.; Merazig, H.; Aouf, N. E. RSC Adv. 2015, 5, 99775-99780.
CrossRef - Bouasla, R.; Berredjem, M.; Aouf, N. E.; Barbey, C. Acta Crystallographica Section E: Structure Reports Online. 2008, 64, o432. Boufas, W.; Dupont, N.; Berredjem, M.; Berrezag, K.; Becheker, I.; Berredjem, H.; Aouf, N. E. J. Mol. Struct. 2014, 1074, 180-185. Berredjem, M.; Winum, J.Y. ; Toupet, L. ; Masmoudi, O. ; Aouf, N. E. ; Montero, J. L. Synth. Commun, 2004, 34, 1653-1662.
CrossRef - Ouarna, S.; K’tir, H.; Lakrout, S.; Ghorab, H. ; Amira, A. ; Aouf, Z. ; Berredjem, M. ; Aouf. N. E. Orient. J. Chem. 2015, 31, 913-919.
CrossRef - Bouchareb, F.; Boufas, W.; Cheloufi, H.; Berredjem, M.; Aouf, N. E. Phosphorus, Sulfur Silicon Relat. Elem. 2014, 189, 587-595.
CrossRef - Berredjem, M.; Bouchareb, F.; Ait Kaki, S.; Dekhil, M.; Aouf, N. E. Arab. J. Chem. 2013, DOI: 10.1016/j.arabjc.2013.01.016.
CrossRef - Bouasla, R.; Berredjem, M.; Hessainia, S.; Berredjem, H.; Aouf, N. E. J. Chem. Chem. Eng. 2011, 5, 1153-1159.
- Grib, I.; Bouzina, A.; Aouf, N. E.; Berredjem, M. Phosphorus, Sulfur Silicon Relat. Elem. 2016, 191, 1086-1091.
CrossRef - Bouzina, A.; Aouf, N. E.; Berredjem, M. Res. Chem. Intermed. 2016, 42, 5993-6002.
CrossRef - Mansouri, R.; Aouf, Z.; Lakrout, S.; Berredjem, M.; Aouf, N. E. J. Braz. Chem. Soc. 2016, 27, 546-550.
- Leighton, T. G. The Acoustic Bubble.Academic Press: London, 1994.
- Flint, E. B.; Suslick, K. S. Science. 1991, 253, 1397-1399.
CrossRef - Suslick, K. S.; Didenko, Y.; Fang, M. M.; Hyeon,T.; Kolbeck, K. J.Philos. Trans. R. Soc. London, Ser. A, 1999.
- Mason, T. J.; Peters, D. Practical Sonochemistry Power Ultrasond Uses and Applications, 2nded.; Ellis Horwood: New York, 2002; Mason, T. J. Ultrason. Sonochem. 2007, 14, 476-483.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









