Synthesis of Tio2 and Sio2 Oxide Systems, Containing Nanoparticles of Palladium and Platinum
Nemeryuk A. M, and Lylina M. M.
Federal State Unitary Enterprise ”State Research Institute of Chemical Reagents and Pure Chemical Substances”(FSUE “IREA”), 107076, Russia, Moscow, Bogorodskiy.
Corresponding Author E-mail: amnamn@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/330206
Article Received on : September 24, 2016
Article Accepted on : February 12, 2017
Obtained oxide systems of titanium and silicon dioxide containing platinum and palladium nanoparticles. As a reducing agent and nanoparticles stabilizer used heteropolyethers, obtained by controlled hydrolysis of titanium and silicon alkoxides in the presence of 1,10-decanediol.
KEYWORDS:Titanium dioxide; platinum nanoparticles; palladium nanoparticles; polyols
Download this article as:| Copy the following to cite this article: Nemeryuk A. M, Lylina M. M. Synthesis of Tio2 and Sio2 Oxide Systems, Containing Nanoparticles of Palladium and Platinum. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Nemeryuk A. M, Lylina M. M. Synthesis of Tio2 and Sio2 Oxide Systems, Containing Nanoparticles of Palladium and Platinum. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31332 |
Introduction
Materials based on titanium dioxide are well known thanks to the pronounced catalytic and photocatalytic properties [1-7]. Presence of metallic nanoparticles formed of platinum group metals in oxide systems based on titanium dioxide, often leads to increased catalytic activity [8-12]. Despite the fact that the production of titanium dioxide is a well-researched and widely practiced process, are of great interest for new approaches to the production of materials based on titanium dioxide, containing platinum metals. Processes occurring in the preparation of such systems may affect the shape, size of the individual grains of oxide, the particle distribution of platinum metals and the strength of contact with the particles of the oxide matrix. Depending upon the morphological characteristics of oxide systems containing titanium dioxide, and platinum group metal particles, it varies within wide limits and selectivity of the intensity of catalytic activity. Were studied processes for the production of oxide systems based on titanium dioxide containing platinum nanoparticles as potential catalysts intended for the intensification of oxidative processes in the gas phase.
For oxide systems based on titanium dioxide and platinum metals nanoparticles was used method, based on the interaction of organic precursor containing titanium and platinum metals at elevated temperatures in organic solvents to form an intermediate organic-inorganic composite [1,2, 9-15].
It is found that the reaction of titanium compounds, such as halides and alkoxides with higher polyols initially formed oligomeric heteropolyethers:
TiX4 + HO(CH2)10OH = ((RO)2Ti-O-(CH2)10-O)n,
X=Cl, i-C3H7, alk
R=HO(CH2)10, alk
The ratio between the titanium and a polyol may vary within wide limits, which leads to products of different polymerization degree.
When using tetraalkoxysilanes formed heteropolyethers may contain siloxane moieties:
((RO)2Ti-O-(CH2)10-O-Si(OR)2-O-(CH2)10-O)n,
R=HO(CH2)10, alk
Typically, the titanium alkoxides derived of lower alcohols dimeric or tetrameric while hindered alcohols alkoxides monomeric [16].
Indeed, by reacting titanium tetraalkoxide with 1,10 – decanediol t without solvent at temperatures of about 100оС a transesterification occurs to form heteropolyethers and alcohol, the amount of which can be measured to determine the completion point[17-19]. When the process temperature exceeds 290oC observed decay with the release of titanium dioxide and organic products among which oxacycloundecane and 1,9-decandiene are found, the formation of these products may be associated with intramolecular dehydration, accompanied by the evolution of titanium dioxide. The maximum yield of these compounds observed at a ratio of tetraalkoxide and1,10 – decanediol 1:2.
 |
Scheme 1 Click here to View scheme |
Heteropolyethers are transparent colorless viscous liquids which are soluble in amide solvents and aromatic hydrocarbons. These compounds are unstable to hydrolysis reaction with water to form insoluble in organic solvents gels, accompanied by the release of 1,10 – decanediol:
((RO)2Ti-O-(CH2)10-O)n + H2O = ((RO)2Ti-O)n + HO(CH2)10OH
When present in the reaction mixture some soluble in organic media platinum or palladium compounds, reduction occurs to form metal platinum or palladium, wherein the stabilizer is a polyol.
Thus during the synthesis occur sequentially heteropolyethers formation processes, reduction of complex salts of palladium and platinum, heteropolyethers hydrolysis to form an insoluble gel or organic-inorganic composite containing platinum metal nanoparticles.
When thermal treatment of organic-mineral composite in an oxidizing atmosphere there is thermo-oxidative degradation of organic fragments and formed oxide systems containing nanoparticles of platinum group metals, is firmly linked with the grains of the oxide matrix.
Oxide system can be prepared by themselves or as a layer applied to a particular substrate, such as titanium dioxide, alumina, zirconia.
As precursor of titanium dioxide titanium alkoxide or complexes of titanium tetrachloride with formic acid amides were used, e.g., TiCl4*2[(CH3)2NCHO] and TiCl4*2[(CH3)PhNCHO] the use of alkoxides is preferable, since there are fewer side reactions. As the platinum metal precursors were used [(PhCH2CN)2Pd]Cl2, formed by the reaction of palladium chloride with phenylacetonitrile, and (NH4)2[PtCl4] this compound was prepared from the NH42[PtCl6] by hydrazine or oxalate reduction. Also used tetramethoxysilane, titanium tetraisopropoxide and 1,10-decanediol. N-Methylpyrollidone, o-xylene were used as reaction medium.
Experimental
All operations were carried out in a moisture- and oxygen- free argon atmosphere. All solvents were dried by standard methods.
Preparation TiO2, comprising palladium nanoparticles
Mix 7.02 g of 1,10-decanediol and 2.9 ml (2.78 g) of titanium tetraisopropoxide and heated in an apparatus equipped with a condenser. When the amount of distilled isopropanol is 1.8 ml (2.3 g), straight condenser is reversed and added 17 ml (15 g) of o-xylene. Heat the solution to reflux and add dropwise a solution of 0.2 g of [(PhCH2CN) 2Pd] Cl2 in 50 ml (44 g) of o-xylene containing 0.05 g of water. The reaction mass takes a dark color, which is associated with the formation of metallic palladium. Gradually the mixture is separated into two layers, the bottom is a viscous dark mass, the upper transparent light-yellow liquid. The lower layer was separated and kept under vacuum at 150оС to constant weight. Product was then calcined at 600°C for 3 hours. Obtained 2.1 g of a dark powder. The content of Pd 4,3%
Preparation oxide system based on TiO2 and SiO2, comprising platinum nanoparticles on the surface of titanium oxide particles
1 g of 1,10-decanediol are heated with stirring until dissolved in 50 ml (39.3 g), isopropanol (IPA). Discontinue heating and add 6.8 ml (7 g), tetramethoxysilane (Si(OCH3)4) и and 0.2 ml (0.3 g) conc. nitric acid and 0.5 ml (0.5 g) of water. The mixture is stirred for 30 minutes. Was added 12.5 mL (12 g) of titanium tetraisopropoxide (i-C3H7O)4Ti. Heat and stirring is continued during the 30 minutes. Then, IPA was evaporated in vacuo. 22.5 ml viscous liquid is obtained . The product solution was mixed with 0,3g [(NH4)2][PtCl4] in 90 ml of N-Methylpyrollidone. After stirring 10 minutes, added in small portions 45 g of TiO2. The mixture was heated with stirring to 202°C. After 5 minutes, the solution changes color from light brown to purplish-black, and after 30 minutes becomes black and brown.
Continue heating at 202° C and more stirring during 2 hours. The hot solution was filtered. The precipitate was washed with water. Prepared dark gray precipitate weighing 61.9 g which was dried under vacuum at 75°C for 2 hours.
After drying, a gray powder, powder weighing 52.3 g was placed in a muffle furnace and calcined for 2 hours at 650°C. After calcination, the powder was light gray mass of 43.6 g of platinum content 0.27%.
Results and Discussion
These materials have been studied by SEM. The resulting images are shown in Figures 1 – 5. Figure 1 shows the image of a material containing palladium nanoparticles in Figures 2 and 3 – platinum nanoparticles.
By thermal decomposition of the organic-inorganic polymer not containing platinum metal particles of titanium dioxide are formed having dimensions of about 100 nm, Figure 4
 |
Figure 1: SEM image of material containing palladium nanoparticles Click here to View figure |
 |
Figure 2: SEM image of material containing platinum nanoparticles Click here to View figure |
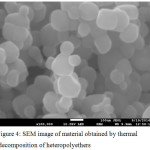 |
Figure 3: SEM image of material containing platinum nanoparticles Click here to View figure |
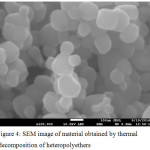 |
Figure 4: SEM image of material obtained by thermal decomposition of heteropolyethers Click here to View figure |
For materials containing platinum, it carried out a detailed study of the elemental composition by fixing the spectrum of the secondary electrons. Visualization of the spectrum of the secondary electrons is shown in Figure 5.
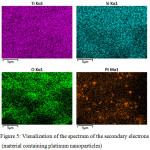 |
Figure 5: Visualization of the spectrum of the secondary electrons (material containing platinum nanoparticles) Click here to View figure |
The composition of the obtained material revealed the platinum nanoparticles, palladium size in the range of 30 to 100 nm, forming a firm contact with grains of titanium oxide platinum particles are smaller, which may be due to differences in the kinetics of reduction. Contact is provided with a thin layer of silicon oxide formed during hydrolysis of tetramethoxysilane. The presence of 1,10 -decandiol and in the reaction medium helps stabilize metal nanoparticles. Aliphatic diols are frequently used in the synthesis of nanoparticles of platinum metals. These compounds reducing salts of, and organic products of oxidation stabilized nanoparticles by inhibiting aggregation. Silicon and titanium alkoxides enter into transesterification reaction with 1,10 – decanediol at elevated temperatures to form oligomeric heteropolyethers. These compounds are capable of reducing the platinum group metals salt to form a platinum or palladium metal nanoparticles and interact with the hydroxyl groups located on the surface of titanium dioxide. As a result, on the surface of titanium dioxide particles formed a layer, firmly binding the platinum nanoparticles. When processing, thermooxidative degradation of organic fragments occurs with the formation of oxide system containing silicon and titanium dioxides, ensuring firm contact of the platinum nanoparticles with the surface of titanium oxide grains. The presence of silica prevents the conversion of anatase to rutile under heat treatment.
Conclusion
A method for producing oxide systems based on titanium dioxide, and platinum, palladium nanoparticles described. Oligomeric heteropolyether is a reducing agent and metal nanoparticles stabilizer. During heat treatment of the obtained organic-inorganic composites formed close contacts between platinum particles and a surface of titanium dioxide grain.
Acknowledgements
Applied researches are carried out with financial support of the state by the Russia Ministry of Education and Science under Grant Agreement No.14.624.21.0006 of September 8, 2014 (Unique identifier for Applied Scientific Researches (project) RFMEFI62414X0006).
References
- Fan X. L., Zhang C. X., Xue H. R., Guo H., Song L. and He J. P. RSC Adv. 2015, 5, 78880-78888.
CrossRef - Ismail A. A. and Bahnemann D. W. Green Chem. 2011, 13, 428-435.
CrossRef - Zhang F., Chen J., Zhang X., Gao W., Jin R., Guan N., Li Y. Langmuir, 2004, 20 (21), 9329–9334.
CrossRef - Ma J., Valenzuela E., Gago A. S., Rousseau J., Habrioux A., and Alonso-Vante N. The Journal of Physical Chemistry 2014, 118 (2), 1111-1117.
- Zare M., Mortezaali A., and Shafiekhani A. The Journal of Physical Chemistry. 2016, 120 (17), 9017-9027.
- Zhang D. Monatsh Chem. 2012, 143, 729.
CrossRef - Huang S., Ganesan P., Jung W. S., Cadirov N. and Popov B. N. ECS Trans. 2010, 33(1), 483-491
- Kameneva, O., Kuznestov, A.I., Smirnova, L.A., Rozes, L., Sanchez, C., Alexandrov, A., Bityurin, N., Chhor, K., and Kanaev, A., J. Mater. Chem., 2005, 15, 3380–3383.
CrossRef - Wu, Ch.-S., J. Appl. Polym. Sci., 2004,. 92,. 3, 1749–1757.
CrossRef - Sanchez, C., Soler-Illia, A.A., Ribot, F., Lalot, T., Mayer, C.R., and Cabuil, V., Chem. Mater., 2001,. 13(10),. 3061–3083.
CrossRef - Huang, S.L., Chin, W.K., and Yang, W.P., Polymer, 2005,. 46,. 1865–1877.
CrossRef - Bradley, D.C. and Millger, H.J., Trans. Faraday Soc., 1966, 62, 2374–2381.
CrossRef - Trabelsi, S., Janke, A., Hässler, R., Zafeiropoulos, N.E., Fornasieri, G., Bocchini, S., Rozes, L., Stamm, M., Gérard, J.-F., and Sanchez, C., Macromolecules, 2005,. 38,. 6068–6078.
CrossRef - Markin, A.V., Yakimovich, N.O., Smirnova, L.A., and Smirnova, N.N., , Polym. Sci., Ser. B, 2008, 50,124–127.
CrossRef - Lionel N., Laberty-Robert C., Rozes L. and Sanchez C. Nanoscale, 2014,6, 6267-6292.
CrossRef - T. J. Boyle, T. M. Alam, E. R. Mechenbier, B. L. Scott,J. W. Ziller, Inorg. Chem. 1997.,36, 32-93 .
- Y. Pérez, I. Hierro, M. Fajardo, A. Otero, Journal of Organometallic Chemistry, 2003., 679(2), 220-228.
CrossRef - Y., I. Hierro, M. Fajardo, , Journal of Organometallic Chemistry, 2012.,717(15), 172-179.
- Y. Pérez, D. Pérez Quintanilla, M. Fajardo, I. Sierra, I. Hierro, Journal of Molecular Catalysis A: Chemical, 2007., 1(2), 227-237.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









