Synthesis, Characterization and Structural Study of New Ethyl Thiourea Compounds and the Preliminary Naked-Eye Sensors for Mercury (Hg) and Argentum (Ag) Metal Ions
Siti Aishah Hasbullah, Syahidatul Munirah Raffik, Nurnadzirah Mat Noh, Emma Izzati Zakariah, Fatimatul Akma Awang Ngah, Hamza Milad Abosadiya and Bohari Yamin
School of Chemical Sciences and Food Technology, Faculty of Science and Technology, Universiti Kebangsaan Malaysia, 43600 Bangi, Selangor, Malaysia. Corresponding Author E-mail: Aishah80@ukm.edu.my
DOI : http://dx.doi.org/10.13005/ojc/330227
Article Received on : October 09, 2017
Article Accepted on : January 01, 2017
Novel ethyl thiourea compounds 1a – 1f have been synthesized in good to high yields. The structure of all compounds was identified using FT-IR, 1H NMR, 13C NMR, and ESI-Mass. The compound 1-ethyl-3-(3-methoxyphenyl) thiourea 1d was obtained in crystalline form by recrystallization from DMSO. However, single crystal X-ray analysis revealed that the 1d molecule crystallized in a monoclinic crystal system with space group of P21/n and the unit cell dimensions are a = 11.0797(7) Å, b = 8.6081(6) Å, c = 11.9698(7) Å, α = 90°, β =104.543(2)°, γ = 90°, Z = 4 and V = 1105.04(12) Å3. The preliminary optical sensor properties for metal ions of 1a – 1f were investigated using naked-eye. The results determined that all compounds have the ability as naked eye sensors for mercury (Hg) and argentum (Ag) metal ions.
KEYWORDS:Synthesis; Structural study; Ethyl Thiourea; Naked-eye Sensors
Download this article as:| Copy the following to cite this article: Hasbullah S. A, Raffik S. M, Noh N. M, Zakariah E. I, Ngah F. A. A, Abosadiya H. M, Yamin B. Synthesis, Characterization and Structural Study of New Ethyl Thiourea Compounds and the Preliminary Naked-Eye Sensors for Mercury (Hg) and Argentum (Ag) Metal Ions. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Hasbullah S. A, Raffik S. M, Noh N. M, Zakariah E. I, Ngah F. A. A, Abosadiya H. M, Yamin B. Synthesis, Characterization and Structural Study of New Ethyl Thiourea Compounds and the Preliminary Naked-Eye Sensors for Mercury (Hg) and Argentum (Ag) Metal Ions. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31471 |
Introduction
Thiourea is similar to urea compound except the oxygen atom in urea is replaced by the presence of sulphur atom in thiourea compound.1 The chemical properties of both compounds are different because of the oxygen and sulphur atoms in urea and thiourea. These atoms have different in electronegativity where oxygen has greater electronegativity compare to sulphur. Many applications can be done on behaviours.2 Thiourea is a chemically interesting compound where it has more than two functional groups in a compound: amino, imino and thiol.3 Thiourea derivatives are mostly crystalline compounds and thermally stable. They can be produced in good yields from available starting material by simple syntheses.3 Thiourea is also one of the most famous studied compounds on metallic electrodes.4 It can absorb to metal surface and the absorption blocks the active sites.4 It is also one of the important industrial chemical products.5 Nowadays, the situation of environmental pollution by heavy metals is one of the critical issues due to the development of modern industries and agriculture.
Many of heavy metals can be dangerous towards human or animal health. Mercury (Hg2+) is one of the most toxic and hazardous between all the heavy metals.6 Mercury can cause cells damage and some diseases related to brain,7 kidney, and central nervous system. These are because the mercury is unable to decay naturally and accumulates in animal and human bodies.8 Silver ions (Ag+) also have long been known to have strong inhibitory and antimicrobial activities.9 Silver nanoparticles are showing very toxic effects on human health and the environment due to the antibacterial activity. Then, chronic exposure to silver also can causes negative effects such as permanent bluish-grey discoloration of the skin (argyria) and eyes (argyrosis). Besides that, it also may produce other toxic effects like liver and kidney damage, irritation of the eyes, skin, respiratory and intestinal tract and changes to blood cell.10 For the above reasons, there is a high demand for tracking these metal ions.11 The aim of the present study was to synthesis thiourea compounds having alkyl group substitute.
Chemical sensor is generally consisting of two units; receptor unit and signalling unit, but in some cases spacer is also used to provide the shape and geometry in two armed molecule.12 Detecting metal cations is one of the great interests to many scientists.13 Thioureas are well known having good affinity towards metals via thiono forup (C=S) make them applicable as ion sensors.14 The nitrogen, oxygen and sulphur atom are donor atoms of thiourea that can donate electrons to form bonding and provide a multitude of bonding possibilities.15,16 The structural characterization of the thiourea derivatives was performed using FTIR, 1H NMR, 13C NMR spectroscopy devices and ESI-MS spectrometry. In order to investigate the sensor properties, the interaction of all compounds with metals in solvent media was investigated using naked-eye sensor.
Materials and Methods
All reactions were performed under conventional reflux method. Chemicals and solvents were purchased from Sigma Aldrich and Acros and used directly without any further purification. Melting points were recorded on the open tube capillary method using Electrothermal 9100 instrument (Electrothermal, England) and are uncorrected. All compounds were analyzed using FTIR Perkin Elmer Model Spectrum GX in the range 400-4000cm-1 using KBr method, 1H and 1C NMR spectrometer model Bruker Advance 400 MHz and Liquid Chromatography Mass Spectroscopy (ToF) model Dionex/Bruker MicroToF Q. Single-crystal X-ray experiments were performed on a Bruker D-QUEST diffractometer (Bruker, AXS Inc., Madison, WI, USA) using graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). The ω-scan measured the intensity data at room temperature. The full-matrix least-squares fit of 25 reflections determines the accurate cell parameters and orientation matrix. Intensity data were collected for Lorentz and polarization effects. Empirical absorption correction was carried out using multi-scan. The structure was solved by direct methods and least-squares refinement of the structure was performed by the SHELXL-2007 program.17 All the non-hydrogen atoms were refined anisotropically. The hydrogen atoms were placed in the calculated positions except those of the terminal nitrogen atoms of thiourea moieties located from Fourier maps and refined isotropically.
General Procedure for Synthesis of Compounds 1a-1f
A solution of ethyl isothiocyanate (0.1394 g, 0.0016 mol) in dichloromethane (20 mL) was added dropwise to a suspension of toluidine 1a, 1b or 1c (0.1714 g, 0.0016 mol) and anisidine 1d, 1e or 1f (0.1968 g, 0.0016 mol) in dichloromethane. The reaction mixture was refluxed for 24 hours and was monitored using thin layer chromatography (TLC) until the reaction was completed. The white precipitate was formed and filtered out. The solid product was purified by recrystallization method using ethanol: dichloromethane mixture.
1a Mp: 81.9 – 83.6 K. IR (KBr), NH: 3193.00 cm-1, C-N: 1371.00 cm-1, C=S: 1245.00 cm-1. 1H NMR (400 MHz, CDCl3-d6) δ (ppm) 1.14 (3H, t J=7.2 Hz, CH3), 2.29 (3H, s, CH3), 3.63 (2H, s, CH2), 5.65 (1H, s, NH), 7.25 (1H, d J=10.8 Hz, ArCH), 7.26 (1H, t J=7.8 Hz, ArCH), 7.28 (1H, t J= 7.8 Hz, ArCH), 7.30 (1H, d J=10. 8 Hz, ArCH), 7.84 (1H, s, NH). 13C NMR (100 MHz, CDCl3-d6) δ (ppm) 14.5 (CH3), 17.8 (CH3), 40.2 (CH2), 127.5-131.8 (4 x ArCH), 135.9 (ArC), 134.4 (ArC), 180.6 (C=S). m/z (ESI) (H+, C10H14N2S requires 195.09)
1b Mp: 84.9 – 85.6 K. IR (KBr), NH: 3011.00 cm-1, C-N: 1355.00 cm-1, C=S: 1148.00 cm-1. 1H NMR (400 MHz, CDCl3-d6) δ (ppm) 1.18 (3H, t J=7.2 Hz, CH3), 2.36(3H, s, CH3), 3.66 (2H, s, CH2), 6.01 (1H, s, NH), 7.30 (2H, d, J=7.2 Hz, ArCH), 7.32 (2H, d J=7.6 Hz, ArCH), 8.20 (1H, s, NH). 13C NMR (100 MHz, CDCl3-d6) δ (ppm) 14.4 (CH3), 21.4 (CH3), 40.3 (CH2), 122.2 (2 x ArCH), 129.9 (2 x ArCH), 136.1 (ArC), 140.4 (ArC), 180.2 (C=S). m/z (ESI) (H+, C10H14N2S requires 195.09)
1c Mp: 97.4 – 98.8 K. IR (KBr), NH: 3198.00 cm-1, C-N: 1317.00 cm-1, C=S: 1249.00 cm-1. 1H NMR (400 MHz, CDCl3-d6) δ (ppm) 1.16 (3H, t, J=7.2 Hz, CH3), 2.36 (3H, s, CH3), 3.64 (2H, s, CH2), 5.95 (1H, s, NH), 7.10 (1H, d, J=7.6 Hz, ArCH), 7.20 (1H, d, J=7.6 Hz, ArCH), 7.32 – 7.33 (2H, m, ArCH), 8.06 (1H, s, NH). 13C NMR (100 MHz, CDCl3-d6) δ (ppm) 14.3 (CH3), 21.1 (CH3), 40.3 (CH2), 128.0 – 130.8 (4 x ArCH), 133.4 (ArC), 137.5 (ArC), 180.4 (C=S). m/z (ESI) (H+, C10H14N2S requires 195.09)
1d Mp: 72.5 – 72.5 K. IR (KBr), NH: 3335 cm-1, C-C aromatic: 1528 cm-1, C=S: 1263 cm-1. 1H NMR (400 MHz, CDCl3-d6) δ (ppm) 1.20 (3H, t, J=7.2 Hz, CH3), 3.67 (2H, sex, J=5.6 Hz, CH2), 3.85 (3H, s, OCH3), 6.11 (1H, s, NH), 6.98 (1H, t, J=7.6 Hz, ArCH), 7.23 (1H, td J=6.4 Hz and 1.6 Hz, ArCH), 7.31 (1H, s, ArCH), 7.52 (1H, s, NH). 13C NMR (100 MHz, CDCl3-d6) δ (ppm) 14.3 (CH3), 40.3 (CH2), 55.7 (OCH3), 112.0 – 124.9 (4 x ArC), 127.5 (ArC), 152.4 (ArC), 180.3 (C=S). m/z (ESI) (Na, C10H14N2OS requires 233.27g/mol)
1e Mp: 112.5 – 117.5 K.IR (KBr) NH: 3343 cm-1, C-C aromatic: 1550 cm-1, C=S: 1284 cm-1. 1H NMR (400 MHz, CDCl3-d6) δ (ppm) 1.19 (3H, t, J=7.2 Hz, CH3), 3.67 (2H, sex, J=5.4 Hz, CH2), 3.80 (3H, s, OCH3), 6.11 (1H, s, NH), 6.78 (1H, dd, J=6.0 Hz and 2.0 Hz, ArCH), 6.74 (1H, t, J=2.0 Hz, ArCH), 6.82 (1H, dd, J=6.0 Hz and 2.4 Hz, ArCH), 7.32 (1H, t, J=8.0 Hz, ArCH), 7.95 (1H, s, NH). 13C NMR (400 MHz, CDCl3-d6) δ (ppm) 14.3(CH3), 40.4 (CH2), 55.5 (OCH3), 110.7-130.9 (4 x ArC), 137.3 (ArC), 160.9 (ArC), 180.1 (C=S). m/z (ESI) (Na, C10H14N2OS requires 233.27 g/mol)
1f Mp: 121.5 – 124.5 K.IR (KBr), NH: 3297 cm-1, C-H: 2988 cm-1 C-C aromatic: 1520 cm-1, C=S: 1289 cm-1. 1H NMR (400 MHz, CDCl3-d6) δ (ppm) 1.15 (3H, t, J=7.2 Hz, CH3), 3.64 (2H, sex, J=5.6 Hz, CH2), 3.82 (1H, s, OCH3), 5.78 (1H, s, NH), 6.93 (1H, d, J=8.8 Hz, ArCH), 7.14 (1H, d, J=8.8 Hz, ArCH), 7.72 (1H, s, NH). 13C NMR (400 MHz, CDCl3-d6) δ (ppm) 14.4 (CH3), 40.2 (CH2), 55.5 (OCH3), 115.2 – 127.7 (4 x ArC), 128.6 (ArC), 158.8 (ArC), 180.8 (C=S). m/z (ESI) (Na, C10H14N2OS requires 233.27g/mol)
General Procedure for Naked-Eye Sensor
For naked-eye sensor detection of metal ions (Fe2+, Pb2+, Hg2+, Ni2+, Zn2+, Ag+, Cu2+, and Sn2+), compounds 1a, 1b and 1d were dissolved in 25 ml dimethyl sulfoxide (DMSO) to form 1 x 10-3 M solution. Metal ion sources were prepared in deionized water to form 1 x 10-3 M stock solution except SnCl2 in DMSO. The color changes were observed by naked eyes and the data were collected.
Results and Discussions
All ethyl thiourea compounds 1a – 1f were synthesized in moderate to high yields from the reaction of ethyl isothiocyanate 2 with isomers of toluidine 3a – 3c and anisidine 3d – 3f in dichloromethane under reflux condition. Scheme 1 outlines the synthesis of ethylthiourea compounds 1a – 1f.
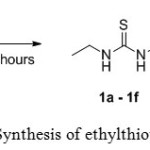 |
Scheme 1: Synthesis of ethylthioureas 1a–1f Click here to View scheme |
FT-IR spectra were taken with KBr pellets between 4000 and 400 cm-1. In the FT-IR spectra of all the synthesized compounds show sharp and strong absorption band in the region of 3011 – 3335 cm-1 and it were attributed to the stretching vibration of NH group, respectively. In addition, synthesized compounds show strong intensity C=S stretching vibration in the 1213 – 1284 cm-1 range. The proton NMR spectrum (1H and 13C) were in agreement with compounds 1a – 1f. The peaks in the region of 7.60 – 8.20 ppm were attributed to NH proton near the aromatic ring. This singlet NH resonance is more de-shielded due to anisotropic effect. The distinctive broad singlets in the range of 5.65 – 6.20 ppm were attributed to NH proton attached to the ethyl terminal ring. The present of electron donating ethyl moieties increased the electron density and shifted the protons of NH to the upfield position. The protons at aromatic ring were assigned at 6.80 – 7.36 ppm. The protons for methyl (1a – 1c) and methoxy (1d – 1f) at aromatic ring exhibited signal as singlet in the range of 2.28 – 2.36 ppm and 3.80 – 3.85 ppm, respectively. The peaks in the region of 1.1 to 3.7 ppm were due to the protons of methyleneand methylof ethyl ring. According to the 13C NMR spectra, the signal of C=S ring was found around 180.2 ppm and indicated the formation of thiourea moiety. The aromatic carbons were observed in the range of 110.7 – 160.9 ppm for all compounds. The methyl (1a – 1c) and methoxy (1d – 1f) carbons for aromatic ring were resonated at 21.0 and 55.5 ppm, respectively. All other carbons at the ethyl terminal ring were resonated in the region of 14.3 – 40.3 ppm. The signal related to [M + H]+ and [M + Na]+ for compounds 1b and 1d in the MS spectrum were observed at 195.09 and 233.27, respectively.
X-ray diffraction study indicates that the compound 1d crystallizes in the monoclinic crystal system with space group of P21/n and the unit cell dimension are a = 11.0797(7) Å, b = 8.6081(6) Å, c = 11.9698(7) Å, α = 90°, β =104.543(2)°, γ = 90°, Z = 4 and V = 1105.04(12) Å3. Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 1493627. Summary of the data collections and details of the structure refinement is given in Table 1.
Table 1: Crystal data and structure refinement of 1-ethyl-3-(3-methoxyphenyl) thiourea 1d
| Crystal parameters | Data/values | |
| Empirical formula | C10H14N2OS | |
| Formula weight | 210.29 | |
| Temperature | 302(2) K | |
| Wavelength λ | 0.71073 Å | |
| Crystal system | Monoclinic | |
| Space group | P21/c | |
| Unit cell dimensions | a = 11.0797(7) Å | α = 90° |
| b = 8.6081(6)Å | β = 104.543(2)°° | |
| c = 11.9698(7) Å | γ = 90° | |
| Volume | 1105.04(12) Å3 | |
| Z | 4 | |
| D, cal (Mg/m3) | 1.264 | |
| Absorption coefficient | 0.263 mm−1 | |
| F(000) | 448 | |
| Crystal dimension (mm3) | 0.44 x 0.37 x 0.29 | |
| Theta range for data collection | 2.89 to 28.40° | |
| Reflections collected | 40490 | |
| Ranges/indices (h,k,l) | -14, 14; -11, 11; -15, 15 | |
| Completeness to theta | 28.40°, 99.6% | |
| Independent reflections | 2753 [R(int) = 0.0791] | |
| Max. and min. transmission | 0.9275 and 0.8929 | |
| Data / restraints / parameters | 2753 / 2 / 137 | |
| Goodness of fit on F2 | 1.100 | |
| R1, wR2 (I≥2σ(I)) | R1 = 0.0580, wR2 = 0.1072 | |
| R1,wR2 indices (all data) | R1 = 0.1024, wR2 = 0.1220 | |
| Largest diff. peak and hole | 0.230 and -0.285 e.Å-3 | |
Fig. 1 shows the molecular structure with numbering scheme of compound 1d which is analogous to the previously reported 1-(2-Hydroxyethyl)-3-(3-methoxyphenyl)thiourea (Choi et al. 2010) except in 1d compound, there is no hydroxyl group bonded to the terminal carbon of ethyl group.
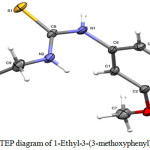 |
Figure 1: ORTEP diagram of 1-Ethyl-3-(3-methoxyphenyl)thiourea 1d Click here to View figure |
The molecule is not planar. However, the thiourea fragment S1/N1/N2/C8/C9 and the benzene ring (C1—C6) are essentially planar with a maximum deviation of 0.003(2) Å for N2 atom from the least square plane of the thiourea fragment. The two planes forming a dihedral angle of 88.54(9)°, which is considerably bigger than that found in 1-(2-Hydroxyethyl)-3-(3-methoxyphenyl)thiourea of 62.57(4)°. Other bond lengths and angles are comparable to those in the analog and lie in normal ranges (Table 2).
Table 2: Selected bond lengths and angles of 1-Ethyl-3-(3-methoxyphenyl)thiourea 1d
| Bond | Length Å | Bond | Angles° |
| S1-C8 | 1.6917(18) | C2-O1-C7 | 118.32(16) |
| O1-C2 | 1.366(2) | C8-N1-C6 | 124.92(16) |
| O1-C7 | 1.420(3) | C8-N2-C9 | 124.91(17) |
| N1-C8 | 1.339(3) | O1-C2-C3 | 115.78(16) |
| N1-C6 | 1.436(2) | O1-C2-C1 | 124.25(18) |
| N2-C8 | 1.326(3) | C5-C6-N1 | 119.26(18) |
| N2-C9 | 1.456(3) | C1-C6-N1 | 119.36(17) |
The intramolecular hydrogen bonds does not exists in the molecule while in the crystal packing the molecules are linked by N1—H1A···S1 and N2—H2A···O1 intermolecular hydrogen bonds (Table 3), to form infinite one-dimensional chains along the c axis (Fig. 2).
Table 3: Hydrogen geometric parameters (Å) of 1-Ethyl-3-(3-methoxyphenyl)thiourea 1d
| D―H….A | D―H | H….A | D….A |
D―H….A |
| N1―H1A…..S1i | 0.866(14) | 2.528(16) | 3.3678(18) | 163.6(16) |
| N2―H2A…..O1ii | 0.865(19) | 2.331(17) | 3.126(2) | 152.9(19) |
Symmetry codes: i= -x,1-y,-z, ii= -x,1-y,1-z
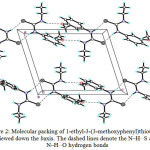 |
Figure 2: Molecular packing of 1-ethyl-3-(3-methoxyphenyl)thiourea 1d viewed down the b axis. The dashed lines denote the N–H…S and N–H…O hydrogen bonds Click here to View figure |
The ability and sensor features of compounds 1a, 1b and 1d were investigated using the naked-eye in DMSO solvent mixture in the presence of metals such as Fe, Pb, Hg, Ni, Ag, Cu and Sn. It was determined that ethylthioureas 1a – 1f did not exhibit naked-eye sensor properties for Fe, Pb, Ni, Cu and Sn metals. However, the compounds exhibited selective sensor behavior for Hg+ and Ag+ metal ions (Fig. 3).
 |
Figure 3: The interaction of compound 1a, 1b and 1d with metal cations Click here to View figure |
Thiourea derivatives contain a relatively basic C=S group that interacts with the metal ions. The C=S bond which was weak and had the resonance structure (Scheme 2) was the dominant resonance contributor.8 The resonance structure will be the nucleophile to attack the metal ions to form a stable complex (Scheme 3).
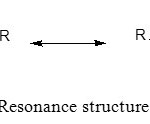 |
Scheme 2: Resonance structure of thiourea
|
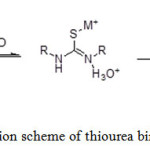 |
Scheme 3: Reaction scheme of thiourea binding with metal ions Click here to View scheme |
Conclusions
In the present work, ethyl thiourea 1a – 1f were prepared from the simple reaction method and are characterised using various spectral techniques. From the X-ray crystallography study of 1d, molecule crystallized in a monoclinic crystal system with space group of P21/n and the unit cell dimensions are a = 11.0797(7) Å, b = 8.6081(6) Å, c = 11.9698(7) Å, α = 90°, β =104.543(2)°, γ = 90°, Z = 4 and V = 1105.04(12) Å3. Prelimanary study showed that ethyl thiourea 1a – 1f have the ability as naked eye sensors for mercury (Hg) and argentum (Ag) metal ions.
Acknowledgement
The authors thank the Ministry of Higher Education of Malaysia and Universiti Kebangsaan Malaysia for the research grant FRGS1/2015/ST01/UKM/02/2 and PRGS/2/2015/SG01/UKM/02/1 for the X-ray facility.
References
- Abdul Fattah, M. A.; Lusta, I. Z.; Zubi, A. E. International Scholarly and Scientific Research & Innovation 2014, 8(2), 116-118.
- Alkan, C.; Tek, Y.; Kahraman, D. Turkish Journal of Chemistry 2011, 35(5), 769-777.
- Mertschenk B.; Beck F.; Bauer W. Weinheim: Wiley-VCh Verlag GmbH & Co. KGaA. 1995.
- Zhao J.; Cui G. International Journal of Electrochemical Science 2011, 6, 4048 – 4058.
- Asieh Y.; Zahra G. Eur. Chem. Bull. 2013, 2(8), 573-575.
- Rurack, K.; Resch-Genger, U. Chemical Society Reviews 2002, 31(2): 116-127.
CrossRef - Jae-Young L.; Boddu Ananda R.; Ji-Yong H.; and Young-A. S. Sensors and Actuators B 2015, 220, 1070–1085.
CrossRef - Zhang, H.; Gu, C. D.; Huang, M. L.; Wang, X. L.; Tu, J. P. Electrochemistry Communications 2013, 35, 108-111.
CrossRef - Berger, T.; Spadaro, J.; Chapin, S.; Becker, R. Antimicrobial Agents and Chemotherapy 1976, 9(2), 357.
CrossRef - Panyala, N. R.; Peña-Méndez, E. M.; Havel, J. J Appl Biomed 2008, 6(3), 117-129.
- Li, X.; Gao, X.; Shi, W.; Ma, H. Chemical Reviews 2013, 114(1), 590-659.
CrossRef - Kumar V.; Kaushik M.P.; Srivastava A.K.; Pratap A.; Thiruvenkatam V.; Guru Row T. N. Analytica Chimica Acta 2010, 663, 77–84.
CrossRef - Valeur, B.; Leray, I. Coordination chemistry reviews 2000, 205(1), 3-40.
CrossRef - Abosadiya, H. M.; Siti Aishah Hasbullah; Bohari M Yamin. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2015, 144, 115-124.
CrossRef - Saeed S.; Rizwan Hussain. Eur. Chem. Bull. 2013, 2(7), 465-467.
- Arslan H.; Duran N.; Borekci G.; Koray O.; Akbay C. Molecules 2009, 14, 519-522.
CrossRef - Sheldrick, G. M. SHELXTL. Version 6.14. Program for Crystal Structure Determination. University of Gottingen: Gottingen, Germany 1997.

This work is licensed under a Creative Commons Attribution 4.0 International License.









