Synthesis Characterization and Analytical Applications of Antimony(III) molybdoarsenate as a Cation–Exchanger and Selective for Pb(II) and Cu(II) Ions
Teena1, Vandana Sharma 2 and Kaushal Kumar3
1Department of Chemistry, Chaudhary Charna Singh University, Meerut, U.P 250002.
2Department of Enviromental Science, Deen Dyal Upadhaya College, University of Delhi 110078.
3Department of Chemistry, Meerut College, Meerut, U.P 250004.
Corresponding Author E-mail: teenaprakash15@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330219
Article Received on : March 18, 2017
Article Accepted on : March 23, 2017
These three component inorganic ion-exchanger was synthesized under different condition. The most thermal and chemical properties of this material is prepared by intermixing solutions of sodium molybdate (0.1 M), Sodium arsenate (0.1 M) and antimony(III) Chloride (0.1 M) solution in different volume ratios at pH-1. The Ion-exchange capacity of all the samples was determined by column process. The selected sample was synthesized in bulk for detailed studies. Its Ion-exchange capacity of synthesized material for Na+ has been found to be 2.30 meq/g. Characterization of the prepared material was done by different parameters like FTIR, TGA Curve, X-ray diffraction, and other studies include pH titration, Kd values, thermal & chemical stabilities. The synthesized ion-exchanger has been demonstrated by achieved binary and water softening separations for different analytical practical uses.
KEYWORDS:Cation-exchanger; pH titration; distribution studies; thermal and chemical stabilities; binary separation; hardness of water
Download this article as:| Copy the following to cite this article: Teena T, Sharma V, Kumar K.Synthesis Characterization and Analytical Applications of Antimony(III) molybdoarsenate as a Cation–Exchanger and Selective for Pb(II) and Cu(II) Ions. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Teena T, Sharma V, Kumar K.Synthesis Characterization and Analytical Applications of Antimony(III) molybdoarsenate as a Cation–Exchanger and Selective for Pb(II) and Cu(II) Ions. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31879 |
Introduction
Ion-exchange may be defined as reversible interchange of ions takes place between two phases is solid and liquid1. Ion-exchange technology is most important versatile technology in the field of separation science. These techniques can be applied to both macro as well as micro analysis for the routine separation. Thus separation is a important part of chemical analysis2. Mostly synthetic ion-exchangers were inorganic materials. These were the synthetic zealites or permutites which were developed by Grans, and other workers for use in water softening process3.In recent years, various zeolites with completely regular crystal structure have been synthesized like zeolite A4.
Inorganic cation-exchanger have drawn the attention to their selectivity, temperature, thermal and chemical stabilities5. Synthesized a two component ion-exchanger antimony(III) silicate of the class of multivalent metal acid salt and its ion-exchange behaviour towards alkali and alkaline earth metal ions was determined by the column technique6. Similarly a large number of three component ion-exchangers like antimony(III) iodostannate7, titanium(IV) tungstoarsenate8, stannic(IV) molybdophosphate9 and zirconium(IV) selenomolybdate10, cerium(IV) iodotungstate11, iron(III) tungstomolybdate12
In the present work is concerned with the new synthesized and characterized three component ion-exchanger. The ion-exchange capacity was determined by using column and pH titration method. Further characterization of the exchanger was done by chemical and thermal stabilities, FTIR, X-ray, and Kd values. The newly synthesized ion-exchanger is found to be possess extensive application in analytical and industrial chemistry. The important applications achieved with the help of Antimony(III) molybdoarsenate are binary separation.
Experimental
Materials and Instrumentation
All chemicals and reagents were used for prepared material. A Digital Toshniwal pH Meter was used for pH measurement. Thermal and equilibrium studies were performed with the help of Tanco’s Electric Rotary Shaker respectively. Samson S-300 D Electronic Balance was used for weighing. Philips Analytical X-ray B.V. Diffractometer and Cary Aglient 630 (ATR module) IR Spectrophotometer were used for XRD and FTIR respectively. All glass wares of borosil make are used throughout experiment research work.
Preparation of Ion-Exchanger Material
Three component ion-exchanger was prepared by mixing 0.1 M antimony(III) chloride aqueous solution gradually to adding an aqueous solution of sodium molybdate (0.1 M) and sodium arsenate(0.1 M) in different volume ratios to get various samples. The pH of resulting precipitates was maintained at 1.0 with the help of dil hydrocloric acid and allowed to stand for twenty four hours at room temperature. The precipitates was filtered, and washed with demineralized water. The material was dried at 40+10C in an oven. The dried material was broken into small granules by placing in demineralized water. The granules were converted into hydrogen form by keeping with M HNO3 solution for twenty four hours at room temperature. The material was then washed with demineralized water to remove the excess acid and finally material dried at 40+10C in an electric oven. Prepared material are known as an exchanger. The results are shown in Table 1.
Table 1: Synthesis of antimony(III) molybdoarsenate
| Sl.No. |
Molar Conc. (M) |
Mixing Ratios | pH | Appearance of Precipitate | IEC (meq/g) | ||
| Sb3+ | MoO42- | AsO32- | |||||
| 123
4 5 6 |
0.10.10.1
0.1 0.1 0.1 |
0.10.10.1
0.1 0.1 0.1 |
0.10.10.1
0.1 0.1 0.1 |
1:1:11:2:21:2:1
2:1:1 2:2:1 1:1:3 |
11111
1 |
Mid cream ppt.Mid cream ppt.Sky blue ppt.
Sky blue ppt. Sky blue ppt. Dark blue ppt. |
0.480.981.69
1.89 1.99 1.12 |
| 7 | 0.1 | 0.1 | 0.1 | 1:3:1 | 1 | Light blue ppt. | 0.79 |
| 8 | 0.1 | 0.1 | 0.1 | 3:1:1 | 1 | Light yellow ppt. | 1.02 |
| 9 | 0.1 | 0.1 | 0.1 | 2:1:3 | 1 | Dark blue ppt. | 2.30 |
| 10 | 0.1 | 0.1 | 0.1 | 3:2:1 | 1 | Light yellow ppt. | 1.12 |
Characterization
Ion-Exchange Capacity
IEC of the different samples was determined by column, taken 0.50 g of the material in H+ form in a each column having glass wool support and washed them, with demineralized water. A molar solution of sodium nitrate was passed through the each column maintaining the flow rate at 8-10 drops/min. The effluents were carefully collected in 250 ml conical flask separately in each case. The H+ ions eluted from the material through column were titrimetrically determined with the help of standard NaOH solution.
The ion–exchange capacity of the material was also determined by monovalent and bivalent, alkali and alkaline earth metals as represented by Table 2.
Table 2: Ion-exchange capacity of antimony(III) molybdoarsenate for different metal cations
|
Sl. No. |
Cation |
Salt Used |
Concentration of Salt Used |
I.E.C. (meq/g) |
Hydrated Ionic Radii (A0) |
| 1234
5 6 |
Li+Na+K+Mg2+
Ca2+ Ba2+ |
LiClNaClKBrMgCl2
CaCl2 BaCl2 |
0.1 M0.1 M0.1 M0.1 M
0.1 M 0.1 M |
2.102.302.471.76
1.89 2.05 |
10.07.95.310.80
9.60 8.80 |
pH Titration
pH titration was performed by the batch method using the method of, Topp and Pepper13. In this method the pH titration of the exchanger in H+ form was performed in NaCl-NaOH system. Eleven equal amount of 0.50 g it the exchanger in each were placed in eleven 250 ml conical flasks separately by the addition of equimolar solutions of NaCl and NaOH in different volume ratios, the final volume of the solution was kept 50 ml so as to maintain the ionic strength constant. Then the intermittent shaking of the mixture was kept at room temperature. The pH titration curve of the exchanger is shown in Figure 1.
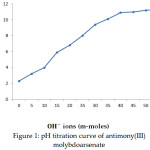 |
Figure 1: pH titration curve of antimony(III) molybdoarsenate |
Thermal Stability
To determine the thermal stability of the exchanger was evaluated by heating it at different temperatures upto 700oC in a muffle furnace for one hour, and then cooled to room temperature in a desiccator. The loss of weight and change in colour of the ion-exchanger was observed in each case and ion-exchange capacity was determined by column method. The results are given in Table 3.
Table 3: Thermal stability of antimony(III) molybdoarsenate
|
Sl.No. |
Dying Temperature (0C) |
Weight of Ion-Exchanger Before Heating (g) |
Change in Colour |
IEC (meq/g) |
|
1 2 3 4 5 6 7 |
1000C 2000C 3000C 4000C 5000C 6000C 7000C |
0.50 0.50 0.50 0.50 0.50 0.50 0.50 |
Dark blue Dark blue Light grey Dark grey Dusky black Black Black |
1.90 1.20 0.81 0.41 0.22 0.10 —- |
Chemical Analysis
To examine the chemical stability of the exchanger was assessed in different mineral acids such as H2SO4, HNO3 and HCl bases such as KOH and NaOH, organic acids such as CH3COOH, HCOOH, 0.50 g of the exchanger was taken in 50 ml of several molar solutions and kept for twenty four hours at room temperature and then filtered and finally dried at 40+10C in an oven. Now the dry material loss of weight of all samples was observed and ion-exchange capacities were determined by column method as represented in Table 4.
Table 4 :Chemical stability of antimony(III) molybdoarsenate
|
Sl.No. |
Solution |
Percentage Weight Loss |
Ion-Exchange Capacity |
|
1 2 3 4 5 6 7 8 9 10 11 12 |
1M HCl2M HCl1M HNO32M HNO31M H2SO42M H2SO4
1M CH3COOH 2M CH3COOH 1M HCOOH 2M HCOOH 2M KOH 2M NaOH |
2 4 0 0 6 12 6 12 8 16 Completely Dissolve Completely Dissolve |
1.50 1.01 2.30 2.28 1.00 0.55 1.10 0.72 0.51 0.26 —– —– |
Distribution Studies
Distribution coefficient for several metal ions in demineralized water was carried out by the batch process14. In this process, 0.50 g portion of the exchanger in H+ form was equilibrated with 25 ml of 0.1 M solutions of different metal ions. The mixture was shaken for three hours with the help of rotary shaker and then to attain complete equilibrium. The determination of metal ions present in the solution was titrated against the standard solution of EDTA. Kd values were calculated using the formula
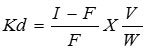
Where I is the intial volume of EDTA solution, F is the final volume of EDTA solution after equilibrated, V is the volume of the metal ion solution (ml) and W is the weight of the ion-exchanger in gram15. The results are summarized in Table 5.
Table 5:Distribution coefficient studies of antimony(III) molybdoarsenate
|
Sl.No. |
Metal Ions |
Form |
Kd (mlg-1) |
|
1 2 3 5 6 7 8 9 10 11 |
Mg(II) Ca(II) Mn(II) Zn(II) Co(II) Cu(II) Cd(II) Ni(II) Pb(II) Bi(II) |
Acetate Carbonate Acetate Acetate Acetate Acetate Chloride sulphate Nitrate Nitrate |
9.98 10.24 7.90 7.79 7.12 12.42 7.89 32.14 11.20 7.03 |
FTIR and X-ray Diffraction
IR studies of the material were recorded between 3800 cm-1 and 800 cm-1. The spectrum shows a broad bands in the 3452.17 region, characteristics of the O-H stretching and bending mode. Finally the spectrum also shows strong and weaks bands at 1618.84 cm-1 and 783.74 cm-1, respectively which indicates the presence of arsenate and molybdate. The band at 783.74 cm-1 shows the presence of metal oxide bond. The spectrum are shown in Figure 2. The X-ray diffraction pattern of the exchanger are given in Figure 3. Data shows a amorphous nature with weak peaks.
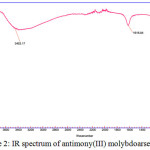 |
Figure 2: IR spectrum of antimony(III) molybdoarsenate |
 |
Figure 3: XRD of antimony(III) molybdoarsenate Click here to View figure |
TGA Curve
The themogram of antimony(III) molybdoarsenate in H+ formwas get it done at Instrumentation centre, IIT Roorkee at a rate of 100C/min. in nitrogen atmosphere from room temperature. The results are shown in Figure 4.
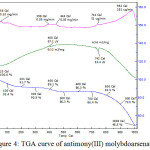 |
Figure 4: TGA curve of antimony(III) molybdoarsenate
|
Separations Achieved
The values of separation factor for different metal ions pairs obtained antimony(III) molybdoarsenate were greater than three and their values are given in Table 6.
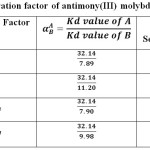 |
Table 6: Separation factor of antimony(III) molybdoarsenate |
Binary Separation of Metal Ions
The separations factors are the guiding measures for the separation. Binary separation of metal ions were achieved are given in Table 7. For binary separations, 0.50 g of the exchanger in H+ form was packed in glass column. The column was washed with demineralized water and then metal ion mixture was poured in column separately. The absorbed metal ions were eluted with appropriate eluents one by one. The flow rate of the effluent was maintained at 1 ml/min throught the elution process16. The effluents were collected separately conical flask and metal ion concentration were determined (Complexometric Titration) against disodium EDTA salt solution using suitable indicators.
Table 7: Binary separation achieved with the help of antimony(III) molybdoarsenate
|
Sl. No. |
Metal Ion Pair |
Amount Loaded (µg) |
Amount Found (µg) |
% Metal Ion Eluted |
% Error |
Total Eution Volume |
Eluent Used |
|
1 |
Ni(II) Cd(II) |
9390 1624 |
9180 1625 |
97.76 100.06 |
-2.240.06 |
40 ml 50 ml |
0.001 M HNO3 0.1 M HNO3+0.5 M NH4OH |
|
2 |
Ni(II) Pb(II) |
9390 2239 |
9390 1920 |
95.95 85.75 |
-5.15-5.25 |
50 ml 40 ml |
0.1 M HClO40.1 M HNO3 |
|
3 |
Ni(II) Mn(II) |
9390 1337 |
8990 1223 |
95.74 91.47 |
-5.26-9.53 |
50 ml 30 ml |
1 M NH4Cl+0.1 M HCl0.1 M HCl |
|
4 |
Ni(II) Mg(II) |
9390 4861 |
9460 4770 |
100.75 98.12 |
0.75-2.88 |
80 ml 70 ml |
1.0 M HNO30.4 M NH4NO3 |
Water Softening
Hardness causing Ca2+ and Mg2+ were also removed with help of the sysnthesized ion-exchanger. column method was used for the removal of ions. The hardness of the water samples was determined by complexometric titration method, in which, Erichrome Black-T was used as an indicator. Hardness causing Ca2+ and Mg2+ loaded in the column were eluted using 0.01 M HClO4 and 1.0 M HNO3aseluents respectively. The results are summerised in Table 8.
Table 8: Removal of Ca2+ and Mg2+ with the help of antimony (III) molybdoarsenate
| Sl.No. |
Metal Ion Pair |
Amount Loaded (µg) |
Amount Found (µg) |
% Metal Ion Eluted |
% Error |
Total Eution Volume |
Eluent Used |
|
1 |
Mg2+ |
4861 |
4800 |
98.74 |
-2.26 |
50 ml |
1.0 M HNO3 |
|
2 |
Ca2+ |
5743 |
5741 |
99.96 |
-1.04 |
50 ml |
0.01 M HClO4 |
Results and Discussion
Different samples of the material in various volume ratios have been synthesized (shown in Table 1). One of the sample was selected for detailed studies. The high ion exchange capacities for mono and divalent metal ions are shown in Table 2. It is evident that the affinity sequence for monvalent metal ions is K+>Na+>Li+ and for divalent ions is Ba2+>Ca2+>Mg2+. The effect of charge and size of the ion on the ion-exchange capacities show that exchanging ion takes place in the hydrated form. The metal ions with smaller hydrated radii easily enter the pores of exchanger, resulting in higher adsorption17 . The pH titration curve was carried out under equilibrium stage for NaCl-NaOH system. The curve was plotted between pH values and OH& ions concentration. The results are shown in Figure 1.
The heating effect on the weight and ion–exchange capacity at various temperatures upto 7000C was carried out. Data shows that the ion-exchange capacity decrease with increase in temperature (Table 3). Antimony(III) molybdoarsenate are less stable in lower concentration of HCl, H2SO4 and CH3COOH, HCOOH. The ion-exchanger was completely dissolve in 2 M KOH and 2 M NaOH solution (Table 4).
IR spectrum was performed at room temperature used by KBr disc method. The results are shown in Figure 2.
X-ray diffraction pattern of antimony(III) molybdoarsenate is amorphous nature, which indicate weak peaks with sharp intensities (Figure 3). Thermogram of prepared material recorded in Figure 4. The water molecule are lost upto 1000C (4.6%) as indicated by the first peak of the curve. Further loss in weight is observed from 4000C to 5000C and the corresponding weight loss in 24.6%. From 500 0C to 9000C, the loss is very slow. i.e. 13.7% at 6000C, 13.6% at 7000C, 17.5% at 8000C & 27.5% at 9000C. At 10080C a rapid decline in weight (51.5%) is noticed.
Distribution behaviour indicate that the exchanger is selective for Ni(II), Cu(II), Pb(II), Ca(II), in compare to another metal ions. Solvent are used in binary separation and water softening 0.1 M HNO3, 0.1 M HNO3, 0.1 M H3PO4, 1 M HClO4, 0.1 M HNO3+0.1 M NH4NO3 it can be used for the Quantitative analysis trace metal ion in environmental purpose. The results are shown in Table 7. binary separation of Ni-Cd, amounts found of nickel and cadmium shows that almost all the nickel metal are eluted while 100% of cadmium ions are eluted showing the percentage error of 0.06%. Similarly Ni-Pb, Ni-Mn and Ni-Mg pairs separation results are satisfactory as nickel is removed 95-100%. These results can be compared with the previously reported binary separation results. Ni-Mg separation achieved with the help of stannic(IV) antimonate18 and tin(IV) tungstate19. The recovery ranged from 98-100% with variation of 2% for repetitive determinations.
Water softening results obtained for antimony(III) molybdoarsenate revealed that the Ca2+ and Mg2+ can be removed to a great extent i.e. 99% and 98.74% respectively with the help of these exchanger. The results are shown in Table 8.
Conclusion
Newly, synthesized cation-exchanger are now the established material in analytical chemistry useful in separation technique. Antimony(III) molybdoarsenate is good ion-exchange capacity and is stable up to a fairly high temperature, and fairly stable in different acidic, basic and organic media. these ion-exchanger possesses selectivity for trace metals such as, Pb, Cd, Ni, Co,Cu. synthetic ion-exchanger are also employed for the binary separation of heavy metals present in aqueous solution.
References
- Subhash Chand, Seema, Teena and Manju, International Transactions in Applied Sciences, 2010, 2 (1), 181-190
- Bhawna A., Shah, Ajay V., Shah and Pathik M., Shah, Iranian Polymer Journal, 2000,15 (10),809-819.
- Virendra Kumar, Shuka I.C., Yadav O.P., J. Indian Chem. Soci., 2015, 91, 825-829.
- Ismail A. A., Mohamed R.M., Ibrahim I.A., Kini G., and Koopman B., Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2010, 366(1-3), 80-87.
CrossRef - Syed Ashfaq Nabi, Amjad M.T., Khan, Indian Journal of Chemistry, 2005, 44(A), 1383-1387.
- Reetha C, Aravindakshan and Janardanan C, Indian Journal of Chemical, 2002, 41(A), 1438-1440.
- Subhash Chand, Teena, Manju, International Transactions in Applied Sciences, 2013, 5 (4), 467-478.
- Varshney K.G., Agarwal K, Agarwal S, Saxena V, Khan A.R., Colloids and Surface,1988, 29, 175.
CrossRef - Qureshi M., Shakeel N A., Rizvi S N A. , Gupta A P., Journal of Indian Chemical Society,1987, 64,15.
- Gupta A P., Verma G L., Saiqa Ikram, Reactive and Functional Polymers,2000, 43, 33-41.
CrossRef - Dhara S., Sarkar S., Basu S., Chattopadhyay P., 2009, 67 (4), 530-534.
- Geetha, Janardhan C., Indian Journal of Chemical Technology, 2006, 13, 185-188.
- Topp N E. and Pepper K W., Journal Chemical society, 1949,3299.
CrossRef - Qureshi M, Varshney K G., and Israeli A H J., Chromatography,1972, 50, 141.
- Virendra Kumar, Shukla I.C., Asian Jouranl of Chemistry, 2017, 29, 559-564.
CrossRef - Teena, Subhash Chand and Sonia, International Journal of Basic and Applied Chemical Sciences 2015, 5 (2), 29-39.
- Nabi S A, Usmaris and Rahman N., Ann. Chim. Fr.1996, 21,521.
- Aditya K. Mishra, Journal of Chemical Technology,2000, 132-136.
- Alpana H. Parikh, Uma V. Chudasama, Indian Journal of Chemistry,2003, 42, 559-563.

This work is licensed under a Creative Commons Attribution 4.0 International License.









