Synthesis and in Silico Analysis of Novel 1,2,4-Triazolylamides as Potential Antifungals
Khushbu Gumber1, Anjali Sidhu1, Vineet K. Sharma2 and Anju Bala3
1Department of Chemistry Punjab Agricultural University, Ludhiana, Punjab, India.
2Department of Plant Pathology Punjab Agricultural University, Ludhiana, Punjab, India.
3Department of Plant Breeding and Genetics Punjab Agricultural University, Ludhiana, Punjab, India.
Corresponding Author E-mail: Khushbugumber8@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330220
Article Received on : February 27, 2017
Article Accepted on : April 05, 2017
The lead hybridization concept was used to synthesize the series of 40 novel compounds having a 1,2,4-triazole moiety allied to various bioactive amines via carboxamide linkage. One pot two step reaction protocol was followed using K2CO3 as novel and efficient catalyst. The titled compounds were made through preliminary screening for antifungal potential against various phytopathogenic fungi. Compounds 1a, 5a, 9a, 5b and 9b showed appreciable fungitoxicity against most of the test fungi. The best result was shown by compound 1a against Drechslera oryzae, which was comparable to the standard triazole fungicide, propiconazole. Lipophilicity (log P) was taken as critical factor that amend the bio-efficacy of the test compounds.
KEYWORDS:1,2,4-Triazole; 4-Amino-1,2,4-triazole; Amide; Amines; Antifungal activity; Phytopathogenic fungi; in silico studies
Download this article as:| Copy the following to cite this article: Gumber K, Sidhu A, Sharma V. K, Bala A. Synthesis and in Silico Analysis of Novel 1,2,4-Triazolylamides as Potential Antifungals. Orient J Chem 2017;33(2) |
| Copy the following to cite this URL: Gumber K, Sidhu A, Sharma V. K, Bala A. Synthesis and in Silico Analysis of Novel 1,2,4-Triazolylamides as Potential Antifungals. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31595 |
Introduction
Complex II inhibitors play an important role in agrochemical fungicide research and have been known for more than 40 years [1]. The amide belongs to this group of inhibitors and has been known in this area since the discovery of carboxiin [2] and class of ortho-substituted phenyl amides. This leading impact of carboxamides as agrochemical fungicides led to further derivatization and commercialization of a variety of fungicidal compounds. Boscalid, Flutolanil, Mepronil and Tiadianil are some commercially available carboxamides (Figure 1) which have marvellous ability to protect certain plants from severe diseases due to their enhanced penetration effects across the cuticle of the plants [3].
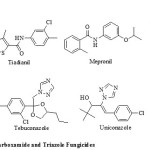 |
Figure 1: Commerialized Carboxamide and Triazole Fungicides |
1,2,4-triazole is another proven lead in the chemical control of fungal diseases as 14-alpha demethylase inhibitors [4-6]. A large variety of them have been derivatized, patented, commercialized and are in use as fungicides for last 30 years. Triadimefon, Tebuconazole, Uniconazole and Propiconazole are some of the well-known available commercial agricultural fungicides (Figure 1). But occurrence of resistance towards these bioactive 1,2,4-triazole fungicides demands novel and chemically diversified approach towards the synthesis of their novel analogues as reduced risk fungicides [7].
Prompted by the augmented effect of lead hybridization on the fungitoxicity profile of novel molecules [8-11], biopotential of 1,2,4-triazole and carboxamide leads as antifungals, along with physically compatible behaviour of amides as crystallization inhibitors for azole fungicides [12], we herein, reported the synthesis and antifungal potential of novel 1,2,4-triazolylamides. The results of fungitoxicity were further rationalized on the basis of in silico toxicity analysis and log P calculations with statistical work-up.
Materials and Methods
Chemicals and Instrumentation
All solvents and reagents were purchased from CDH Company. Solvents used were of analytical reagent grade and purified by standard drying methods [13]. The products were splendidly purified and the purity of all the compounds was checked on a silica gel-G plates and visualization was done using iodine lamp. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance II 400 MHz spectrometer in DMSO-d6 using TMS as an internal standard. The IR spectra were recorded on a Perkin Elmer FT-IR spectrometer using KBr disc. Mass spectra were recorded on a Perkin Elmer Clarus 500 Mass Spectrometer. Melting points of the synthesized molecules were determined in open capillary tubes and are uncorrected.
Chemistry
General Procedure for the Synthesis of (4H-1,2,4-Triazol-4-Ylamino)-(Substituted)Acetamide (1a-10a)
To the corresponding amine (0.02 moles) in dried acetonitrile (10 mL) was added 4 grams of potassium carbonate, and the mixture was stirred and cooled to 0°C. To the cold mixture, chloroacetyl chloride (3 ml, 0.03 moles) was injected slowly under vigorous stirring in a sealed flask with a gas outlet. The stirring was continued maintaining temperature between 0-5°C, till the change in the physical appearance. The formation of the intermediate product was confirmed by the appearance of the new TLC spot using dichloromethane (1:1) as the solvent system. 4-Amino-1,2,4-triazole (1.26g, 0.015 moles) dissolved in dried acetonitrile with few drops of acetic acid (co-solvent) was drop-wise added to the same reaction mixture and stirring was continued at room temperature. The completion of reaction was again confirmed using TLC with modified mobile phase. The 1% (v/v) of acetic acid was used to increase the Rf of the final product. The mixture was filtered to remove the salt obtained as by-product and the solvent was removed under vacuum. The product was washed with diethyl ether and allowed to solidify and recrystallized from ethanol.
2-(4H-1,2,4-Triazol-4-Ylamino)-1-(1H-Benzo[D]Imidazol-1-Yl)Ethanone (1a)
Off White solid, Yield: 52%. Mp 260-262 °C. IR (cm-1): 3431, 2930, 1639, 1275, 1201. 1H NMR (DMSO, 400 MHz): δ 2.0 (s, 1H, NH), 4.18 (s, 2H, CH2), 7.37-7.86 (m, 4H, benzene ring), 8.08 (s, 1H, benzimidazole ring), 8.32 (s, 2H, triazole ring). 13C NMR (DMSO, 400 MHz): δ 137.4, 114.1, 120.2, 123.0, 142.5, 145.9, 51.5, 165.5, 200. ES-MS: m/z 242.09.
2-(4H-1,2,4-Triazol-4-Ylamino)-1-(1H-Imidazol-1-Yl)Ethanone (2a)
White solid, Yield: 44%. Mp 195-197°C. IR (cm-1): 3425, 2935, 1640, 1275, 1215. 1H NMR (DMSO, 400 MHz): δ 2.1 (s, 1H, NH), 4.19 (s, 2H, CH2), 7.26-8.15 (m, 3H, imidazole ring), 8.83 (s, 2H, triazole ring). 13C NMR (DMSO, 400 MHz): 117.6, 130.8, 136.8, 50.5, 145.9, 200. ES-MS: m/z 192.08.
2-(4H-1,2,4-Triazol-4-Ylamino)-N-Phenylacetamide (3a)
Orange translucent solid, Yield: 60%. Mp. 250-251°C. IR (cm-1): 3368, 2926, 1668, 1245, 1160. 1H NMR (CDCl3, 400 MHz): δ 2.0 (s, 1H, NH), 4.18 (s, 2H, CH2), 7.17-7.61 (m, 5H, benzene ring), 8.83 (s, 2H, triazole ring), 5.0 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 121.6, 128.9, 128.4, 121.6, 145.9, 52.4, 168.5. ES-MS: m/z 217.10
2-(4H-1,2,4-Triazol-4-Ylamino)-N-(4-Fluorophenyl)Acetamide (4a)
Light Brown solid, Yield: 54%. Mp. 264-265°C. IR (cm-1): 3378, 2925, 1672, 1235, 1180. 1H NMR (CDCl3, 400 MHz): δ 2.0 (s, 1H, NH), 4.19 (s, 2H, CH2), 7.42-7.80 (m, 4H, benzene ring), 8.85 (s, 2H, triazole ring), 8.43 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 121.6, 128.9, 128.4, 121.6, 145.9, 52.4, 168.5. ES-MS: m/z 235.09
2-(4H-1,2,4-Triazol-4-Ylamino)-N-(3-(Trifluoromethyl)Phenyl)Acetamide (5a)
Reddish orange solid, Yield: 48%. Mp. 270-272°C. IR (cm-1): 3345, 2934, 1662, 1238, 1184. 1H NMR (CDCl3, 400 MHz): δ 2.0 (s, 1H, NH), 4.18 (s, 2H, CH2), 7.37-7.86 (m, 4H, benzene ring), 8.82 (s, 2H, triazole ring), 8.89 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 121.6, 128.9, 128.4, 121.6, 145.9, 52.4, 168.5. ES-MS: m/z 285.08.
2-(4H-1,2,4-Triazol-4-Ylamino)-N-(Pyridin-2-Yl)Acetamide (6a)
Light brown solid, Yield: 45%. Mp. 275-276°C. IR (cm-1): 3465, 3340, 2948, 1668, 1242, 1190. 1H NMR (CDCl3, 400 MHz): δ 2.0 (s, 1H, NH), 4.26 (s, 2H, CH2), 7.20-8.84 (m, 6H, pyridine and triazole ring), 9.26 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 121.6, 128.9, 128.4, 121.6, 145.9, 52.4, 168.25. ES-MS: m/z 218.09.
2-(4H-1,2,4-Triazol-4-Ylamino)-N-(Pyridin-3-Yl)Acetamide (7a)
Orange solid, Yield: 48%. Mp. 277-280°C. IR (cm-1): 3350, 2953, 1674, 1245, 1195. 1H NMR (CDCl3, 400 MHz): δ 2.0 (s, 1H, NH), 4.22 (s, 2H, CH2), 7.40-8.95 (m, 6H, pyridine and triazole ring), 9.18 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 121.6, 128.9, 128.4, 121.6, 145.9, 52.4, 168.13. ES-MS: m/z 218.09.
2-(4H-1,2,4-Triazol-4-Ylamino)-N-(Pyridin-4-Yl)Acetamide (8a)
Dark Pink solid, Yield: 55%. Mp. 275-278°C. IR (cm-1): 3468, 3342, 2957, 1672, 1212, 1192. 1H NMR (CDCl3, 400 MHz): δ 2.0 (s, 1H, NH), 4.22 (s, 2H, CH2), 7.94-8.56 (m, 6H, pyridine and triazole ring), 9.06 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 121.6, 128.9, 128.4, 121.6, 145.9, 52.4, 168.5. ES-MS: m/z 218.09.
2-(4H-1,2,4-Triazol-4-Ylamino)-N-(Naphthalen-1-Yl)Acetamide (9a)
Brownish grey solid, Yield: 45%. Mp. > 300°C. IR (cm-1): 3355, 2918, 1678, 1263, 1210. 1H NMR (CDCl3, 400 MHz): δ 2.0 (s, 1H, NH), 4.28 (s, 2H, CH2), 6.98-8.07 (m, 7H, napthalene ring), 8.81 (s, 2H, triazole ring), 8.98 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 121.6, 128.9, 128.4, 121.6, 145.9, 52.4, 168.5. ES-MS: m/z 267.11.
2-(4H-1,2,4-Triazol-4-Ylamino)-N-(Furan-2-Ylmethyl)Acetamide (10a)
Light brown solid, Yield: 40%, Mp.272-273°C. IR (cm-1): 3364, 2927, 1665, 1242, 1175. 1H NMR (CDCl3, 400 MHz): δ 2.0 (s, 1H, NH), 4.28 (s, 2H, CH2), 5.18 (s, 2H, furfuryl methylene), 7.26-8.65 (m, 3H, furfuryl ring), 8.86 (s, 2H, triazole ring), 9.08 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 121.6, 128.9, 128.4, 121.6, 145.9, 52.4, 171.0. ES-MS: m/z 221.09.
General Procedure For The Synthesis Of (1H-1,2,4-Triazol-1-Ylamino)(Substituted)Acetamide (1b-10b)
To a solution of 4-amino-1,2,4-triazole (1.68 g, 0.02 moles) in dried acetonitrile (10 mL) and few drops of co-solvent acetic acid was added 4 grams of potassium carbonate, and the mixture was stirred and cooled to 0°C in a sealed flask with a gas outlet. To the cold mixture, chloroacetyl chloride (3 ml, 0.03 moles) was added slowly under vigorous stirring maintaining the low temperature. The stirring was continued till the appearance of the new TLC spot with dichloromethane as solvent system. The corresponding amine (0.015 moles) added to the same reaction mixture and stirring was continued at room temperature. The completion of reaction was confirmed using TLC, and the polarity of the solvent was increased with acetic acid (1%) to get the higher Rf of the final product. The mixture was filtered to remove the by-product and the solvent was removed under vacuum. The product obtained was allowed to solidify and washed with diethyl ether to obtain the pure solid and recrystallized from ethanol.
2-(1H-Benzo[D]Imidazol-1-Yl)-N-(4H-1,2,4-Triazol-4-Yl)Acetamide (1b)
Off white solid, Yield: 44%, Mp. 265-266°C. IR (cm-1): 3385, 2950, 1660, 1272, 1190. 1H NMR (CDCl3, 400 MHz): δ 8.0 (s, 1H, NH), 4.62 (s, 2H, CH2), 7.2-8.3 (m, 7H, aromatic rings). 13C NMR (DMSO, 400 MHz): 121.6, 128.9, 128.4, 121.6, 145.9, 52.4, 173.0. ES-MS: m/z 242.09.
2-(1H-Imidazol-1-Yl)-N-(4H-1,2,4-Triazol-4-Yl)Acetamide (2b)
White solid, Yield: 34%, Mp. 190-192°C. IR (cm-1): 3350, 2945, 1664, 1265, 1192. 1H NMR (CDCl3, 400 MHz): δ 9.25 (s, 1H, NH), 4.62 (s, 2H, CH2), 6.72-8.63 (m, 5H, aromatic rings). 13C NMR (DMSO, 400 MHz): 144.7, 173.0, 37.0, 137.8, 119.1, 128.1. ES-MS: m/z 192.08.
2-(Phenylamino)-N-(4H-1,2,4-Triazol-4-Yl)Acetamide (3b)
Light brown solid, Yield: 64%, Mp. 248-250°C. IR (cm-1): 3372, 2948, 1670, 1278, 1195. 1H NMR (CDCl3, 400 MHz): δ 9.25 (s, 1H, NH), 3.55 (s, 2H, CH2), 7.3-8.54 (m, 7H, aromatic rings), 6.24 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 120.8, 129.5, 113.5, 147.6, 57.3, 173.0, 144.8. ES-MS: m/z 217.10.
2-(4-Fluorophenylamino)-N-(4H-1,2,4-Triazol-4-Yl)Acetamide (4b)
Brown solid, Yield: 52%, Mp.265-267°C. IR (cm-1): 3392, 2942, 1637, 1283, 1189. 1H NMR (CDCl3, 400 MHz): δ 8.0 (s, 1H, NH), 3.77 (s, 2H, CH2), 7.01-8.30 (m, 6H, aromatic rings), 6.28 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 118.9, 116.3, 155.7, 116.3, 118.9, 143.2, 57.3, 173.0, 144.8. ES-MS: m/z 235.09.
N-(4H-1,2,4-Triazol-4-Yl)-2-(3-(Trifluoromethyl)Phenylamino)Acetamide (5b)
Orange solid, Yield: 40%, Mp.272-273°C. IR (cm-1): 3387, 2958, 1658, 1269, 1192. 1H NMR (CDCl3, 400 MHz): δ 8.0 (s, 1H, NH), 3.77 (s, 2H, CH2), 6.8-8.3 (m, 6H, aromatic rings), 6.29 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 129.08, 113.5, 131.08, 124.1, 109.3, 147.9, 116.8, 57.3, 173.0, 144.8. ES-MS: m/z 285.08.
2-(Pyridin-2-Ylamino)-N-(4H-1,2,4-Triazol-4-Yl)Acetamide (6b)
Orange red solid, Yield: 56%, Mp. 274-276°C. IR (cm-1): 3389, 2958, 1660, 1272, 1185. 1H NMR (CDCl3, 400 MHz): δ 8.75 (s, 1H, NH), 3.77 (s, 2H, CH2), 6.6-8.3 (m, 6H, aromatic rings), 6.28 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 117.9, 148.1, 138.3, 106.5, 154.2, 57.3, 173.0, 144.8. ES-MS: m/z 218.09.
2-(Pyridin-3-Ylamino)-N-(4H-1,2,4-Triazol-4-Yl)Acetamide (7b)
Orange solid, Yield: 48%, Mp. 280-282°C. IR (cm-1): 3458, 3374, 2968, 1656, 1268, 1182. 1H NMR (CDCl3, 400 MHz): δ 8.0 (s, 1H, NH), 3.77 (s, 2H, CH2), 7.1-8.3 (m, 6H, aromatic rings), 6.28 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 138.8, 124.7, 135.1, 146.0, 118.1, 57.3, 144.8, 173.0. ES-MS: m/z 218.09.
2-(Pyridin-4-Ylamino)-N-(4H-1,2,4-Triazol-4-Yl)Acetamide (8b)
Dark pink solid, Yield: 45%, Mp. 224-276°C. IR (cm-1): 3376, 2964, 1659, 1275, 1189. 1H NMR (CDCl3, 400 MHz): δ 8.76 (s, 1H, NH), 3.77 (s, 2H, CH2), 6.9-8.3 (m, 6H, aromatic rings), 6.28 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 150.2, 107.3, 154.9, 173.0, 57.3, 144.8. ES-MS: m/z 218.09.
2-(Naphthalen-1-Ylamino)-N-(4H-1,2,4-Triazol-4-Yl)Acetamide (9b)
Greyish brown solid, Yield: 58%, Mp. >300°C. IR (cm-1): 3398, 2965, 1672, 1270, 1203. 1H NMR (CDCl3, 400 MHz): δ 8.74 (s, 1H, NH), 3.77 (s, 2H, CH2), 6.9-8.3 (m, 9H, aromatic rings), 6.25 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 1266.1, 125.0, 128.6, 134.3, 124.7, 119.0, 127.6, 109.3, 147.5, 57.7, 173.0, 144.8. ES-MS: m/z 267.11.
2-(Furan-2-Ylmethylamino)-N-(4H-1,2,4-Triazol-4-Yl)Acetamide (10b)
Light brown solid, Yield: 34%, Mp. 278-280°C. IR (cm-1): 3390, 2978, 1665, 1272, 1194. 1H NMR (CDCl3, 400 MHz): δ 8.0 (s, 1H, NH), 3.22 (s, 2H, furfuryl methylene), 3.66 (s, 2H, CH2), 6.2-8.3 (m, 5H, aromatic rings), 2.0 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 142.1, 110.6, 110.3, 148.7, 50.1, 52.4, 173.0, 144.8. ES-MS: m/z 221.09.
General Procedure for the Synthesis of Substituted-(1H-1,2,4-Triazol-1-Yl)Acetamides (1c-10c)
To a solution of different amines (0.02 moles) in dried acetonitrile (20 mL) was added 4 grams of potassium carbonate, and the mixture was stirred and cooled to 0°C. To the cold mixture, chloroacetyl chloride (3 ml, 0.03 moles) was added drop wise under vigorous stirring. The stirring was continued till the appearance of the new TLC spot. 1,2,4-Triazole (1.135 g, 0.015 moles) dissolved in dried acetonitrile with 2-3 drops of acetic acid was added to the same reaction mixture and stirring was continued at room temperature. The completion of reaction was confirmed using TLC, with dichloromethane as solvent system. The mixture was filtered to remove the salt obtained as by-product and the solvent was removed under vacuum. The product so obtained was allowed to solidify and then recrystallized to give the pure product.
1-(1H-Benzo[D]Imidazol-1-Yl)-2-(1H-1,2,4-Triazol-1-Yl)Ethanone (1c)
Off white solid, Yield: 48%, Mp. 196-197°C. IR (cm-1): 2950, 1672, 1278, 1202. 1H NMR (CDCl3, 400 MHz): δ 4.84 (s, 2H, CH2), 7.2-8.4 (m, 7H, aromatic rings). 13C NMR (DMSO, 400 MHz): 53.1, 120.2, 123.0, 114.1, 137.4, 143.8, 151.5, 142.5, 200. ES-MS: m/z 227.08.
1-(1H-Imidazol-1-Yl)-2-(1H-1,2,4-Triazol-1-Yl)Ethanone (2c)
White solid, Yield: 35%, Mp. 187-189°C. IR (cm-1): 2948, 1675, 1265, 1195. 1H NMR (CDCl3, 400 MHz): δ 4.84 (s, 2H, CH2), 7.2-8.4 (m, 5H, aromatic rings). 13C NMR (DMSO, 400 MHz): 51.5, 143.8, 53.6, 136.8, 130.8, 117.6, 200. ES-MS: m/z 177.07.
N-Phenyl-2-(1H-1,2,4-Triazol-1-Yl)Acetamide (3c)
Light brown solid, Yield: 58%, Mp. 247-248°C. IR (cm-1): 3387, 2953, 1674, 1270, 1187. 1H NMR (CDCl3, 400 MHz): δ 5.65 (s, 2H, CH2), 7.1-8.4 (m, 7H, aromatic rings), 10.44 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 143.8, 151.5, 168.2, 55.5, 138.5, 121.6, 128.9, 128.0. ES-MS: m/z 202.09.
N-(4-Fluorophenyl)-2-(1H-1,2,4-Triazol-1-Yl)Acetamide (4c)
Off white solid, Yield: 52%, Mp. 260-262°C. IR (cm-1): 3365, 2936, 1683, 1269, 1184. 1H NMR (CDCl3, 400 MHz): δ 5.65 (s, 2H, CH2), 7.2-8.4 (m, 6H, aromatic rings), 10.45 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 115.7, 162.9, 120.6, 134.1, 168.2, 56.5, 143.8, 151.5. ES-MS: m/z 220.08.
2-(1H-1,2,4-Triazol-1-Yl)-N-(3-(Trifluoromethyl)Phenyl)Acetamide (5c)
Light brown solid, Yield: 48%, Mp. 275-276°C. IR (cm-1): 3386, 2942, 1687, 1273, 1194. 1H NMR (CDCl3, 400 MHz): δ 5.65 (s, 2H, CH2), 7.3-8.4 (m, 6H, aromatic rings), 10.45 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 151.5, 143.8, 55.5, 168.2, 124.9, 129.2, 120.7, 121.2, 43.1, 125.7, 138.8. ES-MS: m/z 270.07.
N-(Pyridin-2-Yl)-2-(1H-1,2,4-Triazol-1-Yl)Acetamide (6c)
Orange solid, Yield: 54%, Mp. 315-316°C. IR (cm-1): 3318, 2928, 1643, 1282, 1195. 1H NMR (CDCl3, 400 MHz): δ 5.65 (s, 2H, CH2), 7.2-8.4 (m, 6H, aromatic rings), 10.61 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 146.7, 124.4, 138.7, 115.8, 151.8, 168.2, 55.5, 143.8, 151.5. ES-MS: m/z 203.08.
N-(Pyridin-3-Yl)-2-(1H-1,2,4-Triazol-1-Yl)Acetamide (7c)
Dark pink solid, Yield: 45%, Mp. 313-314°C. IR (cm-1): 3300, 2923, 1637, 1287, 1178. 1H NMR (CDCl3, 400 MHz): δ 5.60 (s, 2H, CH2), 7.4-9.2 (m, 6H, aromatic rings), 10.01 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 43.6, 126.7, 146.2, 140.4, 117.8, 168.2, 55.5, 143.8, 151.5. ES-MS: m/z 203.08.
N-(Pyridin-4-Yl)-2-(1H-1,2,4-Triazol-1-Yl)Acetamide (8c)
Dark pink solid, Yield: 48%, Mp. 315-316°C. IR (cm-1): 3325, 2934, 1656, 1273, 1190. 1H NMR (CDCl3, 400 MHz): δ 5.60 (s, 2H, CH2), 8.0-8.6 (m, 6H, aromatic rings), 10.44 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 143.8, 151.5, 55.5, 168.2, 155.3, 109.0, 150.2. ES-MS: m/z 203.08.
N-(Naphthalen-1-Yl)-2-(1H-1,2,4-Triazol-1-Yl)Acetamide (9c)
Light brown solid, Yield: 45%, Mp. 345-346°C. IR (cm-1): 3324, 2926, 1668, 1273, 1196. 1H NMR (CDCl3, 400 MHz): δ 5.65 (s, 2H, CH2), 6.9-8.4 (m, 9H, aromatic rings), 10.01 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 143.8, 151.5, 55.5, 168.2, 135.2, 105.3, 127.6, 119.0, 128.6, 126.0, 125.1, 121.0, 124.7, 134.3. ES-MS: m/z 252.10.
N-(Furan-2-Ylmethyl)-2-(1H-1,2,4-Triazol-1-Yl)Acetamide (10c)
Off white solid, Yield: 35%, Mp. 272-274°C. IR (cm-1): 3354, 2932, 1676, 1262, 1175. 1H NMR (CDCl3, 400 MHz): δ 5.60 (s, 2H, CH2), 5.18 (s, 2H, CH2), 6.2-8.4 (m, 9H, aromatic rings), 8.87 (s, 1H, NH). 13C NMR (DMSO, 400 MHz): 143.8, 55.3, 151.5, 166.9, 37.1, 145.8, 110.4, 110.6, 142.1, 145.8. ES-MS: m/z 206.08.
General Procedure for the Synthesis of 2-(1H-1,2,4-Triazol-1-Ylamino)-N-(Substituted)Acetamide (1d-10d)
To a solution of 1,2,4-triazole (1.38 g, 0.02 moles) in dried acetonitrile (10 mL) with few drops of acetic acid, was added 4 grams of potassium carbonate, and the mixture was stirred and cooled to 0°C. To the cold mixture, chloroacetyl chloride (3 ml, 0.05 moles) was added drop wise under vigorous stirring. The stirring was continued till the appearance of the new TLC spot, with dichloromethane as the solvent system. After the appearance of new spot was added the different amines (0.015 moles) and stirring was continued at room temperature. The completion of reaction was again confirmed using TLC, with 1% (v/v) acetic acid/dichloromethane mixture as solvent system. The mixture was filtered to remove the salt so obtained as by-product and the solvent was removed under vacuum. The product so obtained was allowed to solidify and recrystallized to obtain the pure product.
2-(1H-Benzo[D]Imidazol-1-Yl)-1-(1H-1,2,4-Triazol-1-Yl)Ethanone (1d)
Light Brown solid, Yield: 45%, Mp. 250-251°C. IR (cm-1): 2945, 1695, 1278, 1184. 1H NMR (CDCl3, 400 MHz): δ 4.84 (s, 2H, CH2), 7.4-8.7 (m, 7H, aromatic rings). 13C NMR (DMSO, 400 MHz): 51.4, 110.0, 119.9, 123.0, 134.2, 143.0, 143.8, 144.1, 154.8, 200. ES-MS: m/z 227.22.
2-(1H-Imidazol-1-Yl)-1-(1H-1,2,4-Triazol-1-Yl)Ethanone (2d)
White solid, Yield: 38%, Mp. 185-186°C. IR (cm-1): 2937, 1685, 1287, 1192. 1H NMR (CDCl3, 400 MHz): δ 4.84 (s, 2H, CH2), 6.7-8.7 (m, 5H, aromatic rings). 13C NMR (DMSO, 400 MHz): 49.0, 119.1, 128.1, 137.8, 143.0, 152.8, 200. ES-MS: m/z 177.16.
2-(Phenylamino)-1-(1H-1,2,4-Triazol-1-Yl)Ethanone (3d)
Light Brown solid, Yield: 56%, Mp. 252-253°C. IR (cm-1): 3362, 2920, 1674, 1276, 1195. 1H NMR (CDCl3, 400 MHz): δ 4.17 (s, 2H, CH2), 6.28 (s, 1H, NH), 6.7-8.7 (m, 7H, aromatic rings). 13C NMR (DMSO, 400 MHz): 50.6, 143.0, 152.8, 147.6, 113.5, 129.5, 120.8, 200. ES-MS: m/z 202.21.
2-(4-Fluorophenylamino)-1-(1H-1,2,4-Triazol-1-Yl)Ethanone (4d)
Off white solid, Yield: 48%, Mp. 266-267°C. IR (cm-1): 3350, 2934, 1683, 1275, 1137. 1H NMR (CDCl3, 400 MHz): δ 4.17 (s, 2H, CH2), 6.28 (s, 1H, NH), 7.0-8.7 (m, 6H, aromatic rings). 13C NMR (DMSO, 400 MHz): 50.6, 143.2, 152.8, 143.2, 118.9, 116.3, 156.7, 200. ES-MS: m/z 220.20.
1-(1H-1,2,4-Triazol-1-Yl)-2-(3-(Trifluoromethyl)Phenylamino)Ethanone (5d)
Light brown solid, Yield: 45%, Mp. 281-282°C. IR (cm-1): 3367, 2928, 1689, 1234, 1185. 1H NMR (CDCl3, 400 MHz): δ 4.17 (s, 2H, CH2), 6.28 (s, 1H, NH), 6.8-8.7 (m, 6H, aromatic rings). 13C NMR (DMSO, 400 MHz): 50.6, 143.0, 152.8, 147.9, 109.3, 124.1, 131.8, 113.5, 129.8, 116.8, 200. ES-MS: m/z 270.21.
2-(Pyridin-2-Ylamino)-1-(1H-1,2,4-Triazol-1-Yl)Ethanone (6d)
Dark Pink solid, Yield: 40%, Mp. 312-313°C. IR (cm-1): 3346, 2948, 1696, 1275, 1185. 1H NMR (CDCl3, 400 MHz): δ 4.17 (s, 2H, CH2), 7.39 (s, 1H, NH), 6.6-8.7 (m, 6H, aromatic rings). 13C NMR (DMSO, 400 MHz): 50.0, 106.5, 117.9, 138.3, 143.0, 148.1, 152.8, 154.2, 200. ES-MS: m/z 203.20.
2-(Pyridin-3-Ylamino)-1-(1H-1,2,4-Triazol-1-Yl)Ethanone (7d)
Orange brown solid, Yield: 48%, Mp. 314-315°C. IR (cm-1): 3348, 2956, 1692, 1272, 1189. 1H NMR (CDCl3, 400 MHz): δ 4.17 (s, 2H, CH2), 6.28 (s, 1H, NH), 7.1-8.7 (m, 6H, aromatic rings). 13C NMR (DMSO, 400 MHz): 50.6, 143.0, 152.8, 118.1, 124.7, 135.5, 138.8, 146.0, 200. ES-MS: m/z 203.20
2-(Pyridin-4-Ylamino)-1-(1H-1,2,4-Triazol-1-Yl)Ethanone (8d)
Dark pink solid, Yield: 45%, Mp. 313-314°C. IR (cm-1): 3356, 2963, 1685, 1265, 1194. 1H NMR (CDCl3, 400 MHz): δ 4.17 (s, 2H, CH2), 6.28 (s, 1H, NH), 6.9-8.7 (m, 6H, aromatic rings). 13C NMR (DMSO, 400 MHz): 50.6, 107.3, 143.0, 150.2, 152.8, 200. ES-MS: m/z 203.20
2-(Naphthalen-1-Ylamino)-1-(1H-1,2,4-Triazol-1-Yl)Ethanone (9d)
Purple grey solid, Yield: 44%, Mp. 343-344°C. IR (cm-1): 3386, 2972, 1703, 1285, 1202. 1H NMR (CDCl3, 400 MHz): δ 4.17 (s, 2H, CH2), 6.28 (s, 1H, NH), 6.9-8.7 (m, 9H, aromatic rings). 13C NMR (DMSO, 400 MHz): 51, 109.3, 119.0, 124.7, 125.0, 126.0, 127.6, 128.6, 134.3, 143.0, 147.5, 152.8, 200. ES-MS: m/z 252.27.
2-(Furan-2-Ylmethylamino)-1-(1H-1,2,4-Triazol-1-Yl)Ethanone (10d)
Off white solid, Yield: 40%, Mp. 270-271°C. IR (cm-1): 3342, 2928, 1686, 1268, 1183. 1H NMR (CDCl3, 400 MHz): δ 3.66 (s, 2H, CH2), 3.7 (s, 2H, CH2), 2.0 (s, 1H, NH), 6.2-8.7 (m, 5H, aromatic rings). 13C NMR (DMSO, 400 MHz): 45.7, 49.2, 110.4, 110.6, 142.1, 143.0, 148.7, 152.8, 200. ES-MS: m/z 206.20.
In Silico Analysis
Toxicity Analysis: Toxtree v2.6.6 is an open-source software application used to analyse the toxic effect of different molecules. In order to find out the toxic hazards of all the synthesized compounds, two dimensional models of the compounds were first converted into its simplified molecular-input line-entry system (SMILES format) using the online SMILE converter. Then SMILES code was simply put into the Chemical identifier row available in the Toxtree software, to get the toxic characters of the molecule.
Log P prediction: Molinspiration, a web based software was used for calculating log P values. It involved the use of two computer assisted steps. Firstly, the two dimensional structure of the molecule was drawn in Chemdraw Ultra 10.0 module and the output was saved as .mol file. The .mol files were then converted into SMILES code using the online converter and put in the workspace of molinspiration interface to obtain the predicted log P values.
Evaluation of in Vitro Antifungal Activity
The in vitro antifungal activity of all the title compounds were evaluated against seven phytopathogenic fungi viz. Alternaria alternata, Blumeria graminis, Drechslera oryzae, Fusarium moniliforme, Puccinia striiformis, Puccinia triticina, and Ustilago tritici which are often encountered. The isolates of phytopathogenic fungi were provided by the department of Plant Pathology of the Punjab Agricultural University. The results were compared with the standard fungicide Propiconazole (1-[[2-(2,4-dichlorophenyl)-4-propyl-1,3-dioxolan-2-yl]methyl]-1H-1,2,4-triazole) obtained from the laboratory stock. The in vitro effective molar concentrations of the compounds were determined by Spore germination inhibition technique. For assays, the title compounds to be tested were dissolved in 10 ml of distilled water with small amount of surfactant, Tween 20, wherever necessary, and serial dilutions were made as per the requirement. The incubation temperature and time followed for different fungi were as reported in the literature. All of our susceptibility tests were performed three times by each antifungal agent and the mean of three replicates were subjected to probit analysis for calculating median inhibition concentration (EC50) required to prevent the growth of test spores.
Statistical Analysis
The IBM SPSS statistics 20 software was used to perform the correlation analysis to confirm the relationship between the the log P and EC50 values. The relationship between the two was then assessed by fitting a linear regression model to obtain an equation of prediction between the dependent variable (EC50 value) and the independent variable (log P) by the least square method. The equation is formally expressed as follows:
Y = A + BX
where, Y is a dependent variable, X represents the independent variable, A and B are the regression coefficients determined by the least square analysis. All the results were compiled at 5% level of significance (P < 0.05).
Results and Discussion
Chemistry
Earlier reports from our laboratory on 1,2,4-triazole derivatives endorsed the favourable influence of lead hybridization on the fungitoxic profile against various phytopathogenic fungi [9, 11]. In continuation of this work, on the synthesis of novel antifungal agents by lead hybridization approach, we have extended our synthetic horizon to the synthesis of 1,2,4-triazolylamides along with heterocyclic/aryl amines in a single molecule.
Perusal of literature revealed that, synthesis of amides involved the use of organic bases and water work-up to obtain the pure products [14-17] but, these methods were not considered to be appropriate with the use of triazoles as reactants. Both 1,2,4-triazole and 4-amino-1,2,4-triazole are highly hydrophilic in nature that led to their insolubility in most of the organic solvents. So, as an alternative pathway to overcome this drawback, we followed one pot two step reaction protocol, using soild potassium carbonate support. Acetonitrile was used as solvent, with few drops of acetic acid as co-solvent, for dissolving 1,2,4-triazole amines. The purity and homogeneity of the products were confirmed by single spot thin layer chromatography. After completion of the reaction, the by-products were removed by filtration. The crude solids obtained after evaporation of solvent were purified by recrystallization from ethanol. The spectral data (IR, 1H NMR, 13C NMR and Mass spectroscopic analysis) of all the synthesized compounds were correlated with the proposed structure, which confirmed the formation of the compounds. General reaction protocol is represented by Scheme 1 and Scheme 2.
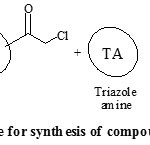 |
Scheme 1: Synthetic procedure for synthesis of compound (1a-10a) and (1c-10c) Click here to View Scheme |
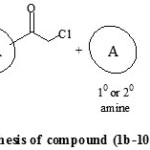 |
Scheme 2: Synthetic procedure for synthesis of compound (1b-10b) and (1d-10d) Click here to View Scheme |
 |
Table Click here to View table |
Antifungal Activity
The spore germination inhibition technique [20] was followed to carry out the antifungal evaluation of all the synthesized compounds and the results were recorded in terms of EC50 values (effective molar concentration at which 50% growth of the test fungus is inhibited). The antifungal activity of the test compounds against the various phytopathogenic fungi viz. Alternaria alternata, Blumeria graminis, Drechslera oryzae, Fusarium moniliforme, Puccinia striiformis, Puccinia triticina and Ustilago tritici were presented in Table 1. Propiconazole (1-[[2-(2,4-dichlorophenyl)-4- propyl-1,3-dioxolan-2-yl]methyl]-1H-1,2,4-triazole) was used as a positive control.
Comparative evaluation of 4-amino-1,2,4-triazole and 1,2,4-triazole derivatives showed the edge of 4-amino-1,2,4-triazole derivatives in the bioactivity profile. Most of the 1,2,4-triazole derivatives were inactive, and direct association of the carbonyl group (-CO-) with the ring made a neutral impact on the activity. Therefore, it can be clinched that the 4-amino-1,2,4-triazole ring can be better used for further development of the amide derivatives. It was further supported by the fact that most of the commercialized carboxamide fungicides (Figure 1) involve the use of primary amine derivatives.
All the compounds had shown the moderate activity when compared with the positive control. The EC50 values revealed that the compounds with a naphthyl ring in the structure (9a, 9b, 9c and 9d) showed good results among the individual classes owing to the higher lipophilicity of these derivatives.
The presence of benzimidazole moiety also inflicted positive effects on the overall results as supported by Bai et al. [21]. On the other hand there is no specific bioactivity of the compounds with imidazole and 1,2,4-triazole ring in the structure and this may be due to very low lipophilicity (negative log P) of the compounds, because of two highly hydrophilic rings in a single structure. Among aniline derivatives (3a, 4a, 5a; 3b, 4b, 5b; 3c, 4c, 5c; 3d, 4d, 5d) the fluoro substitution in the rings had positive influence on the antifungal activity [9, 11] of the compounds and these results were also in consonance with the lipophilicity factor. The similar results favouring the fluorination in the molecule were reported by Tang and co-workers who reported fluorine substituted amidotriazoles with favourable activites [22]. Presence of pyridine ring did not induce favourable inhibition of the germination of the fungal spores. The position of attachment of the ring to the parent structure (6a, 7a, 8a; 6b, 7b, 8b; 6c, 7c, 8c; 6d, 7d, 8d) also resulted in insignificant variation in the bio-efficacy of the molecules.
Table 1: Antifungal potential of the synthesized compounds against various phytopathogenic fungi EC50 (µ moles/ml)
|
Compd |
Log P | A. alternata | B. graminis | D. oryzae | F. moniliforme | P. striiformis | P. triticina |
U. tritici |
| 1a | 0.28 | 0.78 | 0.51 | 0.18 | 0.72 | 0.41 | 0.58 | 0.53 |
| 2a | -1.22 | 1.72 | 1.83 | 1.72 | 1.39 | 1.57 | 1.56 | 1.48 |
| 3a | 0.39 | 1.15 | 0.82 | 0.75 | 0.69 | 0.68 | 0.84 | 0.72 |
| 4a | 0.55 | 0.92 | 0.5 | 0.62 | 0.76 | 0.73 | 0.56 | 0.56 |
| 5a | 1.26 | 0.68 | 0.47 | 0.41 | 0.18 | 0.34 | 0.89 | 0.45 |
| 6a | -0.51 | 1.56 | 2.05 | 2.1 | 1.83 | 1.97 | 2.06 | 1.65 |
| 7a | -0.68 | 1.68 | 1.85 | 1.95 | 1.73 | 1.68 | 1.61 | 1.8 |
| 8a | -0.90 | 1.76 | 1.96 | 1.96 | 1.55 | 1.62 | 1.79 | 1.85 |
| 9a | 1.55 | 0.68 | 0.57 | 0.56 | 0.56 | 0.9 | 0.82 | 0.68 |
| 10a | -0.52 | 1.54 | 1.69 | 1.49 | 1.13 | 1.61 | 1.34 | 1.48 |
| 1b | -0.28 | 0.92 | 0.8 | 0.42 | 0.61 | 0.78 | 0.68 | 0.66 |
| 2b | -1.78 | 2.15 | 1.97 | 1.98 | 1.91 | 1.93 | 1.93 | 1.85 |
| 3b | 0.02 | 1.35 | 0.96 | 0.68 | 0.93 | 0.96 | 1.15 | 0.69 |
| 4b | 0.19 | 1.15 | 0.53 | 1.11 | 0.73 | 0.64 | 0.84 | 0.54 |
| 5b | 0.89 | 0.79 | 0.46 | 0.84 | 0.76 | 0.52 | 0.68 | 0.43 |
| 6b | -0.88 | 1.82 | 1.91 | 1.95 | 2.05 | 1.73 | 1.83 | 1.75 |
| 7b | -1.05 | 1.85 | 1.85 | 1.85 | 1.45 | 1.66 | 1.68 | 1.68 |
| 8b | -1.27 | 1.85 | 1.83 | 1.83 | 1.69 | 1.63 | 1.54 | 1.31 |
| 9b | 1.18 | 0.68 | 0.68 | 0.65 | 0.65 | 0.51 | 0.77 | 0.89 |
| 10b | -1.02 | 1.81 | 1.78 | 1.66 | 1.58 | 1.45 | 1.63 | 1.65 |
| 1c | 0.52 | 1.12 | 0.7 | 1.45 | 1.1 | 0.58 | 0.57 | 0.68 |
| 2c | -0.98 | 2.15 | 2.15 | 2.01 | 2.06 | 2.03 | 2.16 | 1.8 |
| 3c | 0.26 | 1.43 | 1.45 | 1.46 | 0.99 | 1.65 | 1.17 | 1.46 |
| 4c | 0.43 | 1.57 | 1.26 | 1.65 | 2.03 | 1.68 | 0.96 | 1.04 |
| 5c | 1.14 | 0.87 | 1.25 | 1.25 | 0.84 | 1.46 | 0.78 | 0.75 |
| 6c | -0.64 | 2.16 | 2.13 | 2.07 | 2.03 | 2.14 | 2.18 | 1.95 |
| 7c | -0.81 | 2.24 | 2.23 | 2.13 | 2.15 | 2.18 | 2.12 | 2.15 |
| 8c | -1.03 | 2.17 | 2.16 | 2.1 | 2.14 | 2.11 | 2.14 | 2.12 |
| 9c | 1.42 | 0.78 | 0.58 | 0.65 | 0.85 | 0.79 | 0.85 | 0.87 |
| 10c | -0.78 | 2.05 | 1.89 | 1.98 | 1.97 | 1.88 | 2.2 | 2.18 |
| 1d | 0.52 | 0.88 | 1.45 | 1.35 | 1.28 | 1.47 | 0.98 | 0.87 |
| 2d | -0.98 | 1.86 | 1.97 | 1.88 | 1.82 | 2.16 | 2.15 | 2.03 |
| 3d | 0.82 | 1.05 | 1.45 | 1.46 | 1.47 | 1.38 | 1.56 | 1.43 |
| 4d | 0.98 | 0.98 | 1.26 | 1.34 | 1.48 | 1.5 | 0.91 | 0.6 |
| 5d | 1.69 | 0.76 | 0.64 | 0.64 | 0.74 | 0.69 | 0.89 | 0.48 |
| 6d | -0.08 | 1.97 | 1.87 | 1.62 | 2.01 | 1.8 | 2.12 | 1.58 |
| 7d | -0.25 | 2.05 | 2.15 | 2.05 | 2.04 | 2.05 | 2.16 | 2.04 |
| 8d | -0.47 | 2.1 | 2.23 | 2.1 | 2.13 | 2.15 | 2.2 | 1.79 |
| 9d | 1.98 | 0.49 | 0.5 | 0.86 | 0.74 | 0.8 | 0.58 | 0.71 |
| 10d | -0.22 | 1.94 | 1.89 | 1.96 | 0.97 | 1.6 | 1.78 | 1.78 |
| Propiconazole* | 3.64 | 0.17 | 0.15 | 0.13 | 0.15 | 0.18 | 0.2 | 0.18 |
*Standard fungicide against A.alternata, B. graminis, D. oryzae, F. moniliforme, P. striiformis, P. triticina, and U. tritici.
Statistical Analysis
The individual correlation analysis made between log P and EC50 values against various phytopathogenic fungi indicated the negative relationship between the dependent variable (EC50 values) and the independent variable (log P) with corresponding R-values reported in Table 2. A linear regression model was set up to predict the EC50 values from log P for all the test fungi with generalized equation of prediction given in Table 2. All the results were found to be statistically significant at 5% level of significance (P<0.05).
Table 2: Statistical Analysis (P<0.05)
| S.No. | Fungus | Correlation Coefficient (R) | Regression Equation |
| 1. | A. alternata | -0.881 | EC50 = 1.443-0.462(log P) |
| 2. | B. graminis | -0.812 | EC50 = 1.415-0.486(log P) |
| 3. | D. oryzae | -0.767 | EC50 = 1.423-0.438(log P) |
| 4. | F. moniliforme | -0.732 | EC50 = 1.347-0.399(log P) |
| 5. | P. striiformis | -0.733 | EC50 = 1.389-0.403(log P) |
| 6. | P. triticina | -0.773 | EC50 = 1.388-0.432(log P) |
| 7. | U. tritici | -0.784 | EC50 = 1.283-0.432(log P) |
Conclusion
The forty new compounds with active triazole scaffold having equivalent toxicity to the commercial fungicides were synthesized by clean, convenient and efficient method and the molecules were evaluated for their antifungal activity. The rationale of the activity was tried to be made with the lipophilicity of the synthesized molecules. Less or no significant antifungal activity was observed and it is attributed to the poor lipophilicity of the synthesized compounds as otherwise the triazole and the carboxamide derivatives are established leads in the field of fungicides. Thus, it was considered that the bio-efficacy of these molecules can be increased with technique of increasing the lipid solubility of the compounds, so that the effective molecules may more effectively penetrate in the fungal cell. The work related to this is under process and will be presented soon.
Conflict of Interest
We declare that we have no conflict of interest.
References
- Wu, Z.; Hu, D.; Kuang, J.; Cai, H.; Wu, S.; Xue, W. Molecules 2012, 17, 14205-14218.
CrossRef - Carter G.A.; Huppatz J.L.; Wain R.L. Ann. Appl. Biol. 1976, 84, 33.
- Qin, X.; Yu, H.; Liu, J.; Dai, H.; Bing, G.; Qin, Z.; Zhang, X.; Wang, T.; Fang, J. Bioorg. Med. Chem. 2009, 2, 201-210.
- Zan, X.I.; Lai, L.H.; Ji, G.Y.; Zhon, Z.X. J. Agric. Food. Chem. 2002, 50, 3757-3760.
- Schermerhorn, P.G.; Golden, P.E.; Krynitsky, A.J.; Leimkuehler, W.M. JAOAC international 2005, 88, 1491-1502.
- Dutzmann, S.; Stenzel, K.; Jautelat, M.; US patent 6306850 B1, 2001.
- Chai, X.; Zhang, J.; Cao, Y.; Zou, Y.; Wu, Q.; Zhang, D.; Jiang, Y.; Sun Q.; Bioorg. Med. Chem. Lett., 2010 (In Press).
- Sidhu, A.; Khushbu; Kumar, V. Agric. Res. J. 2015, 52, 42-46.
- Sidhu, A.; Kukreja, S. Arab. J. Chem., 2015, (In Press).
- Gumber, K.; Sidhu, A.; Kumar, V. Russian J. Appl. Chem. 2015, 88, 2065-2073.
CrossRef - Kukreja, S.; Sidhu, A.; Sharma, V.K. Res. Chem. Int. 2016, 42, 1-16.
- Rochling, A.; Reizlein, K.; Baur, P. US patent 8124564 B2, 2012.
- Williams, B.D.G.; Lawton, M. J. Org. Chem. 2010. (In Press).
- Servusova, B.; Eibinová, D.; Doležal, M.; Kubíček, V.; Paterová, P.; Peško, M.; Kráľová, K. Molecules 2012, 17, 13183-13198.
- Yellappa, S.; Naveen, M.H.; Giriyapura, R.V.; Doyijode, B.A.K. J. Korean Chem. Soc., 2013, 57, 241-245.
- Hranjec, M.; Sovi, I.; Ratkaj, I.; Pavlovi, G.; Ili, N.S.; Valjalo, L.; Paveli, K.S.; Paveli, S.K.; Karminski-Zamola, G. Eur. J. Med. Chem. 2013, 59, 111-119.
CrossRef - Darsi, S.S.P.K.; Nagamani, K.S.; Devi, B.R.; Naidu, A.; Dubey, P.K.; Der Pharma Chemica 2011, 3, 35-38.
- Puratchikody, A.; Doble, M.; Ramalakshmi, N. J. Pharm. Res. 2012, 5, 340-342.
- Ahsan, M.J.; Samy, J.G.; Khalilullah, H.; Nomani, M.S.; Saraswat, P.; Gaur, R.; Singh, A. Bioorg. Med. Chem. Lett. 2011, 21, 7246-7250.
CrossRef - Devi, T.R.; Chhetry, G.K.N. J. Sci. Res. Pub. 2012, 2, 1-4.
- Bai, Y.B.; Zhang, A.L.; Tang, J.J.; Gao, J.M.; J. Agric. Food Chem. 2013, 61, 2789-2795.
CrossRef - Tang R.; Jin L.; Mou C.; Yin J.; Bai S.; Hu D.; Wu J.; Yang S.; Song B.; Chem. Cent. J. 2013, 7, 30-36.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









