Synthesis and Anti-Inflammatory Activity of Hydrazones Bearing Biphenyl Moiety and Vanillin Based Hybrids
A. L. V. Kumar Reddy1, 2 and Niren E Kathale1
1Department of Chemistry, Sardar Patel Mahavidyalaya, Chandrapur, Gondwana University, Gadchiroli, Maharashtra, India.
2Department of Uranium Oxide Plant, Nuclear Fuel Complex, ECIL Post, 500062, Hyderabad, Telangana State, India.
Corresponding Author E-mail: alvkumar2016@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330250
Article Received on : March 08, 2017
Article Accepted on : April 05, 2017
The intense research work in the field of medicinal chemistry has enhanced the significance of Biphenyl moiety as pharmacologically important compound. Some of the compounds bearing Biphenyl moiety possess important medicinal properties like antihypertensive and calcium channel blocker, anti-inflammatory, diuretic, anti-diabetic activity, antipsychotic and anxiolytic activity. The present article describes the synthesis of novel benzohydrazides and 2-phenylbenzohydrazides bearing Biphenyl moiety and vanillin hybrid. The synthesized compounds (31-40) were characterized by 1H NMR, Mass and IR spectroscopic techniques and were evaluated for anti-inflammatory activity by carrageenan paw edema method. The results of the study revealed that compounds 39 and 40 (bearing 2-phenylacetohydrazides) showed maximum anti-inflammatory activity while the compounds 31, 32, 33, 34 and 38 bearing benzohydrazides displayed moderate anti-inflammatory activity.
KEYWORDS:Biphenyl; Vanillin; Hydrazones; Synthesis; Anti-inflammatory
Download this article as:| Copy the following to cite this article: Reddy A. L. V. K, Kathale N. E. Synthesis and Anti-Inflammatory Activity of Hydrazones Bearing Biphenyl Moiety and Vanillin Based Hybrids. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Reddy A. L. V. K, Kathale N. E. Synthesis and Anti-Inflammatory Activity of Hydrazones Bearing Biphenyl Moiety and Vanillin Based Hybrids. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=32300 |
Introduction
The advancement of novel chemotherapeutic agent is an imperative and demanding task for the medicinal chemist. Many research programs are aimed towards design and synthesis of new drugs. Research on new substances possessing better antimicrobial activity has fascinated significant attention due to continuous increase in the resistance of microbes to the obtainable drugs. Further, the infection caused by such microorganism pretense severe confronts to medicinal scientists and required for development of efficient drugs.
The intense research work in the field of medicinal chemistry has enhanced the significance of Biphenyl moiety as pharmacologically important compound. Some of the compounds bearing Biphenyl moiety possess important medicinal properties like antihypertensive and calcium channel blocker1,2, anti-inflammatory3, diuretic4, anti-diabetic5 activity, antipsychotic and anxiolytic activity6. Some of the Biphenyl hydrazide-hydrazone are known to exhibit very good antimicrobial activity7,8.
Vanillin (4-hydroxy-3-methoxybenzaldehyde), a dietary flavoring agent, has been reported to show antioxidant and anti-mutagenic activities, and has also been proved to be an anticarcinogen against a variety of chemical and physical agents914. Besides its flavor qualities, vanillin exhibits the antimicrobial potential and has been used as a natural food preservative15. In old medicinal literature, vanilla was described as a remedy for fevers16,17.
Hydrazones consisting of azometine –NH-N=CH- proton signify an imperative class of compounds for a therapeutic drug development program in the branch of medicinal chemistry. These compounds possess varied biological and pharmacological properties such as anti‑inflammatory, antihelmintic, cardio protective, antiviral, antimicrobial, antischistosomiasis, analgesic, , anticancer, antiplatelet, anticonvulsant, anti‑tubercular, antifungal, antiprotozoal, antimalarial, anti‑trypanosomal, etc.18-22. In recent years, a number of hydrazone derivatives have been developed and evaluated for their antibacterial activity23, 24.
Combination of different pharmacophores in the same molecule may perhaps guide for the development of new compounds in the drug development program in order to attain a higher biological activity. Therefore the combination of Biphenyl, Vanilin and hydrazone type compounds might offer new effectual drugs against multidrug resistant microbial infections.
Inspired by the various pharmacological activities of the above discussed moieties and in continuation to our research program on acetohydrazide-hydrazone derivatives25, we report here in the synthesis, characterization and anti-inflammatory activity of novel benzohydrazides and 2-phenylacetohydrazide-hydrazone derivatives (31-40).
Results and Discussion
Chemistry
The approach for the synthesis of novel hydrazones (31-40) bearing biphenyl moiety and vanillin hybrid is illustrated in Scheme 1. Esterification of aromatic carboxylic acids (1-5) and substituted 2-phenyl acetic acids (6-10) was accomplished in presence of catalytic quantity of conc;sulphuric in ethanol at reflux for 12-14h resulted in the formation of corresponding ethylbenzoates (11-15) and ethyl-2-phenylacetates (16-20). Treatment of these ethylesters (11-20) with hydrazine-hydrate in ethanol at reflux for 8-10 h produced the corresponding benzohydrazides (21-25) and 2-phenylbenzohydrazides (16-20). On the other hand, condensation of vanillin A and the biphenyl intermediate B 25 in presences of potassium in acetonitrile at 85-90oC resulted in the formation of key intermediate aldehyde C in 88% yield25. Coupling of aldehyde C with benzohydrazides (21-25) in ethanol at reflux for 1h gave the corresponding hydrazide-hydrazone derivatives (31-35) in quantitative yields, while the preparation of acetohydrazide-hydrazone (36-40) was reported by us recently. The structural determination of the synthesized benzohydrazides 31-35 was established by spectroscopic techniques like 1H NMR, IR and mass.
The structure of the key aldehyde intermediate C was confirmed by the presence of signal in 1H NMR at 9.82 ppm as a singlet with one proton integration corresponds to the presence of carbonyl group, this was further confirmed by the presence of characteristic peak in the region 1739 cm-1 in IR spectra. The m/z peak at 337.0 in the mass spectra determines the desired molecular formulae. On the other hand, the 1H NMR of ‘Benzoic acid [4-(4′-fluoro-biphenyl -4-ylmethoxy)-3-methoxy-benzylidene]-hydrazide’ is characterized as follows, the proton signals at 11.74 ppm and 8.38 ppm as singlets is assigned to the groups –CO-NH- and –HC=N- respectively and the aromatic proton signals appeared in the expected region. The methylene and the methoxy groups appeared at 5.20 ppm (-OCH2-) and 3.85 ppm (-OCH3) as singlets. The peaks in the IR spectra at 3236, 1647 and 1548 cm-1 corresponds to –C=O (str), C=N (str) and –NH (def) respectively. The mass spectra of the compound showed, m/z 455.1 (M+1) and is in agreement with the desired molecular formulae. Similarly, the structural elucidation of the remaining benzohydrazide-hydrazone derivative (31-35) was characterized as described above. The structural characterization of the acetohydrazide-hydrazones derivatives (36-40) was discussed by us in our previous published article25.
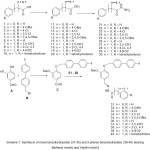 |
Scheme 1: Synthesis of novel benzohydrazides (31-35) and 2-phenyl benzohydrazides (36-40) bearing Biphenyl moiety and Vanillin hybrid |
Experimental conditions
a) conc; H2SO4, Ethanol, reflux, 10-12h; b) Hydrazine-hydrate, Ethanol, reflux, 8-12h; c) B, K2CO3, Acetonitrile, 85-90oC, 2h; d) 21-25 and 26-30, ethanol, reflux, 2-3h.
Anti-inflammatory activity
The synthesized benzohydrazide-hydrazones and 2-phenylacetohydrazide-hydrazone were evaluated for anti-inflammatory activity at 10 mg/Kg po by carrageenan paw edema method and the results are tabulated in Table 1. From table 1, it is observed that compounds 35, 36, 37 (bearing 2-phenylacetohydrazides) showed good anti-inflammatory activity and compounds 39 and 40 (bearing 2-phenylacetohydrazides) showed maximum anti-inflammatory activity while the compounds 31, 32, 33, 34 and 38 bearing benzohydrazides displayed moderate anti-inflammatory activity. In general, with in the series of the synthesized hydrazones, compounds bearing 2-phenylacetohydrazides exhibited pronounced anti-inflammatory activity when compared to 31, 32, 33, 34 bearing benzohydrazides.
Table 1: Anti-inflammatory activity of synthesized hydrazide-hydrazone derivatives 31-40
|
Treatments |
1 hr |
2 hr |
3 hr |
|
Carrageenan Control |
0.98 ± 0.09 |
1.25 ± 0.12 |
2.55 ± 0.12 |
|
31 |
0.46 ± 0.18 |
0.52 ± 0.17 |
0.58 ± 0.04 |
|
32 |
0.48 ± 0.15 |
0.56 ± 0.15 |
0.62 ± 0.22 |
|
33 |
0.36 ± 0.07 |
0.46 ± 0.20 |
0.68 ± 0.078 |
|
34 |
0.40 ± 0.17 |
0.58 ± 0.12 |
0.66 ± 0.18 |
|
35 |
0.50 ± 0.16 |
0.72 ± 0.22 |
0.82 ± 0.17 |
|
36 |
0.55 ± 0.28 |
0.76 ± 0.16 |
0.88 ± 0.24 |
|
37 |
0.58 ± 0.10 |
0.78 ± 0.12 |
0.90 ± 0.20 |
|
38 |
0.46 ± 0.18 |
0.64 ± 0.12 |
0.76 ± 0.21 |
|
39 |
0.68 ± 0.13 |
0.84 ± 0.15 |
0.98 ± 0.20 |
|
40 |
0.72 ± 0.20 |
0.89 ± 0.14 |
1.00 ± 0.21 |
|
|
|||
|
Diclofenac sodium(10mg/kg) |
0.75 ± 0.12 |
0.95 ± 0.12 |
1.16 ± 0.11 |
Experimental Section
Materials and Methods
The solvents were purified according to standard procedures prior to use and all commercial chemicals were used as received. For thin-layer chromatography (TLC) analysis, Merck pre-coated Plates (silica gel 60 F254) were used and spots were visualized under UV light. Merck silica gel 60 (230-400 mesh) was used for flash column chromatography and the eluting solvents are indicated in the procedures. Melting point (mp) determinations were performed by using Mel-temp apparatus and are uncorrected. 1H NMR spectra were recorded in Varian MR-400 MHz instrument. Chemical shifts are reported in δ parts per million (ppm) downfield from tetramethylsilane (TMS) as internal standard reference and the signals were reported as s (singlet), d (doublet), dd (doblet of doblet), t (triplet), q (quartet), m (multiplet) and coupling constants are measured in Hz. The mass spectra were recorded on Agilent ion trap MS and Infrared (IR) spectra were recorded on a Perkin Elmer FT-IR spectrometer.
General Method for the Preparation of Ethylbenzoates (11-15) and Ethyl-2-Phenylacetates (16-20)
Benzoicacids (1-5, 2g, 16.40 mmol) and 2-phenylaceticacid (6-10, 2g, 14.69 mmol) was dissolved in ethanol (25 mL) and then catalytic quantity of concentrated sulphuric acid was added and refluxed for 10-12h. Ethanol was concentrated up to 80% and diluted with ethylacetate (50 mL) followed by water (25 mL). The organic layer was washed with saturated NaHCO3 solution (4 X 20 mL), water and brine solution. The separated organic layer was dried on sodium sulphate, filtered and concentrated to obtain the corresponding ethylbenzoates (11-15) and ethyl-2-phenylacetates (16-20) in 75-80% yield. The isolated compounds were utilized in the next step without any further purification.
General Method for the Preparation of Benzohydrazides (21-25) and 2-Phenylbenzohydrazides (26-30)
Ethylbenzoates (11-15, 1.5g, 9.98 mmol) and ethyl-2-phenylacetates (16-20, 1.5g, 9.13 mmol) were dissolved in ethanol and then hydrazine-hydrate (99%) was added and reflux for 8-12h. Ethanol was concentrated and the resultant residue was poured in ice cold water and stirred for 15 -20 min, the solids that were thrown out was filtered at the pump and dried to obtain the corresponding benzohydrazides (21-25) and 2-phenylbenzohydrazides (26-30) in 80-82% yield.
Benzohydrazide (21)
Pale yellow solid; Yield: 80%; M.p.: 125-126oC; 1H NMR (DMSO-d6, 400 MHz): δ 9.74 (br.s, 1H), 7.82 (d, J = 6.0 Hz, 2H), 7.52-7.44 (m, 3H), 2.28 (br.s, 2H); ESI-MS: m/z 137.0 (M+H)+.
4-Methoxybenzohydrazide (22)
White solid; Yield: 88%; M.p.: 118-119oC; 1H NMR (DMSO-d6, 400 MHz): δ 9.60 (s, 1H), 7.66 (d, J = 6.4 Hz, 2H), 6.96 (d, J = 6.4 Hz, 2H), 4.40 (s, 2H), 3.80 (s, 3H); ESI-MS: m/z, 167.2 (M+H)+.
4-Chlorobenzohydrazide (23)
Off white solid; Yield: 82%; M.p.: 160-162oC; 1H NMR (CDCl3, 400 MHz): δ 9.86 (s, 1H), 7.85 (d, J = 6.8 Hz, 2H), 7.52 (d, J = 8.8 Hz, 2H), 4.52 (s, 2H); ESI-MS: m/z, 169.1 (M+H)+.
3,5-Dichlorobenzohydrazide (24)
White solid; Yield: 88 %; M.p.: 128-129oC; 1H NMR (DMSO-d6, 300 MHz): δ 9.98 (s, 1H), 7.82 (s, 1H), 7.78 (s, 1H), 4.57 (br.s, 2H); ESI-MS: m/z, 203.1 (M+1)+.
3, 4, 5-Trimethoxybenzohydrazide (25)
Off white solid; Yield: 85 %; M.p.: 158-160oC; 1H NMR (CDCl3, 400 MHz): δ 9.50 (s, 1H), 6.42 (s, 1H), 4.45 (br.s, 2H), 3.84 (s, 3H), 3.80 (s, 6H).
2-Phenylacetohydrazide (26)
Off-white solid; Yield; 85%; M.p: 116-117oC; 1H NMR (400 MHz, CDCl3): δ 7.10 (d, J = 6.8 Hz, 1H), 6.82 (d, J = 6.8 Hz, 2H), 6.70 (d, J = 6.6 Hz, 1H0, 5.80 (d, J = 6.6 Hz, 1H), 5.30 (br.s, 1H), 3.56 (s, 2H).
2-(3,5-Dimethylphenyl)acetohydrazide (27)
Pale brown solid; Yield: 88%; M.p.: 94-95oC; 1H NMR (400 MHz, CDCl3): δ 6.90 (s, 1H), 6.82 (s, 2H), 6.70 (br.s, 1H), 3.90 (br.s, 2H), 3.50 (s, 2H), 2.40 (s, 6H).
2-(2-Chloro-4-Fluorophenyl)Acetohydrazide (28)
White solid; Yield: 82%; M.p.: 118-119oC; 1H NMR (400 MHz, CDCl3): δ 7.38-7.36 (m, 1H), 7.18-7.14 (m, 1H), 7.02-6.97 9m, 1H), 6.70 (br.s, 1H), 3.90 (br.s, 2H), 3.60 (s, 2H).
2-(4-Nitrophenyl)Acetohydrazide (29)
Yellow solid; Yield: 88%; M.p.; 167oC; 1H NMR (400 MHz, CDCl3): δ 8.20 (d, J = 3.0 Hz, 2H), 7.42 (d, J = 2.6 Hz, 2H), 6.70 (br.s, 1H), 3.90 (br.s, 2H), 3.60 (s, 2H).
2-(2,3-Dihydrobenzofuran-5-Yl)Acetohydrazide (30)
White solid; Yield: 85%; M.p.: 112-113oC; 1H NMR (400 MHz, CDCl3): δ 7.10 (s, 1H), 6.94 (d, J = 7.2 Hz, 1H), 6.78 (d, J = 7.2 Hz, 1H), 6.62 (br.s, 1H), 4.58 (t, J = 5.8 Hz, 2H), 3.84 (br.s, 2H), 3.42 (s, 2H), 3.18 (t, J = 5.8 Hz, 2H).
Preparation Of 4-[(4′-Fluorobiphenyl-4-Yl)Methoxy]-3-Methoxybenzaldehyde (C)
A mixture of vanillin (A) (2g, 13.14 mmol) and potassium carbonate (2.18g, 15.77 mmol) in acetonitrile (25 mL) was stirred at room temperature and then added compound B (3.66g, 13.80 mmol) in five portions. The reaction mixture was heated to 85-90oC for 2 h. Acetonitrile was concentrated and the residue was poured into ice-cold water and stirred for 15-20 min, the obtained solids were filtered at the pump and dried to obtain compound to obtain compound C. Yellow solid; Yield: 88%; M.p: 98-99oC; IR (KBr): υmax 3021, 2976, 2836, 2736, 1739, 1669, 1584, 1498, 1461, 1422, 1369, 1263, 1224, 1132, 1026, 986, 869, 810, 727, 683, 643 cm-1; 1H NMR (500 MHz, DMSO-d6): δ 9.82 (s, 1H), 7.78-7.72 (m, 4H), 7.58 (d, J = 8.0 Hz, 1H), 7.52 (d, J = 8.0 Hz, 2H), 7.40 (s, 1H), 7.28 (t, J = 8.8 Hz, 2H), 7.22 (d, J = 8.4 Hz, 1H), 5.22 (s, 2H), 3.82 (s, 3H); ESI-MS: m/z, 337.0 (M-1)+;
General Experimental Procedure for the Synthesis of Benzohydrazide-Hydrazone Derivatives (31-35)
A stirred solution of ethanol (2 mL) containing compound C (0.74 mmol) and the corresponding benzohydrazides 21-25, 0.74 mmol) was heated to reflux for 2-3h. The reaction mixture was cooled to room temperature and the solids that were thrown out were filtered, washed with pet-ether and dried to isolated precipitated solids and washed with pet-ether, to obtain the pure compounds 31-35 in 82-85% yield.
Benzoic Acid [4-(4′-Fluoro-Biphenyl -4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 31
White solid; Yield: 82%; M.p.: 113-115oC; IR (KBr): υmax 3236, 3024, 2971, 1647, 1603, 1548, 1504, 1460, 1420, 1370, 1273, 1228, 1171, 1136, 1067, 1049, 999, 958, 904, 862, 810, 756, 693, 654 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 11.74 (s, 1H), 8.38 (s, 1H), 7.90 (d, J = 7.8 Hz, 2H), 7.75-7.67 (m, 4H), 7.62-7.50 (m, 5H), 7.38-7.13 (m, 5H), 5.20 (s, 2H), 3.85 (s, 3H); ESI-MS: m/z, 455.1 (M+1)+;
4-Methoxy-Benzoicacid[4-(4′-Fluoro-Biphenyl-4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 32
Off white solid; Yield: 84%; M.p.: 120-121oC; IR (KBr): υmax 3232, 3020, 2958, 2878, 2838, 1644, 1603, 1548, 1504, 1462, 1421, 1372, 1269, 1233, 1174, 1138, 1029, 999, 958, 905, 841, 808, 759, 674 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 11.62 (s, 1H), 8.38 (s, 1H), 7.90 (d, J = 7.8 Hz, 2H), 7.75-7.67 (m, 4H), 7.54 (d, J = 8.0 Hz, 2H), 7.37-7.30 (m, 3H), 7.27-7.12 (m, 2H), 7.06 (d, J = 9.0 Hz, 2H), 5.19 (s, 2H), 3.85 (s, 3H), 3.83 (s, 3H); ESI-MS: m/z, 485.3 (M+1)+;
4-Chloro-Benzoic Acid [4-(4′-Fluoro-Biphenyl-4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 33
Pale yellow solid; Yield: 85%; M.p.: 130-131oC; IR (KBr): υmax 3552, 3466, 3072, 3024, 2978, 2044, 2877, 2833, 1644, 1599, 1522, 1503, 1461, 1421, 1369, 1274, 1230, 1236, 1097, 1061, 1001, 959, 902, 843, 810, 754, 673 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 11.80 (s, 1H), 8.38 (s, 1H), 7.93 (d, J = 8.5 Hz, 2H), 7.70-7.50 (m, 9H), 7.38-7.14 (m, 4H), 5.20 (s, 2H), 3.85 (s, 3H); ESI-MS: m/z, 489.0 (M+1)+;
3,5-Dichloro-Benzoic Acid [4-(4′-Fluoro-Biphenyl-4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 34
Pale brown solid; Yield:85%; M.p.: 103-108oC; IR (KBr): υmax 3460, 3218, 3069, 3019, 2974, 2877, 2836, 1739, 1660, 1604, 1562, 1507, 1460, 1424, 1370, 1273, 1229, 1106, 1063, 1006, 951, 864, 808, 753, 688 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 11.89 (s, 1H), 8.36 (s, 1H), 7.92 (d, J = 4.5 Hz, 3H), 7.88-7.73 (m, 4H), 7.52 (d, J = 6.2 Hz, 2H), 7.36-7.14 (m, 5H), 5.20 (s, 2H), 3.85 (s, 3H); ESI-MS: m/z, 523.0 (M+1)+;
3,4,5-Trimethoxy-Benzoic Acid [4-(4′-Fluoro-Biphenyl-4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 35
Grey solid; Yield: 84%; M.p.: 94-95oC; IR (KBr): υmax 3217, 3005, 2956, 2839, 1643, 1586, 1505, 1459, 1416, 1369, 1338, 1271, 1229, 1131, 1061, 1004, 961, 918, 854, 815, 759, 699, 626 cm-1; 1H NMR (300 MHz, DMSO-d6): δ 11.60 (s, 1H), 9.85 (s, 1H), 7.75-7.67 (m, 4H), 7.55 (d, J = 8.1 Hz, 2H), 7.38 (s, 2H), 7.33-7.13 (m, 5H), 5.20 (s, 2H), 3.86 (s, 9H), 3.32 (s, 3H); ESI-MS: m/z, 545.1 (M+1)+;
General Experimental Procedure for the Synthesis of Acetohydrazide-Hydrazone Derivatives (36-40)25
To a stirred solution of compound 8 (0.30 mmol) in ethanol was added arylacetohydrazides 26-30, 0.30 mmol) and refluxed for 2-3h. The reaction mixture was cooled to room temperature and filtered the precipitated solids and washed with pet-ether, to obtain the pure compounds 9a-e. Yields of the products varied between 90 and 98%.
Phenyl-Acetic Acid [4-(4′-Fluoro-Bi Phenyl-4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 36
White solid; Yield: 84%; M.p.: 113-114oC; IR (KBr): υmax 3208, 3046, 2938, 2878, 1668, 1597, 1548, 1507, 1457, 1409, 1371, 1339, 1262, 1230, 1186, 1127, 1083, 992, 944, 813, 697 cm-1; 1H NMR (500 MHz, DMSO-d6): 11.50 (* 11.22, s, 1H), 8.18 (* 7.90, s, 1H0, 7.74-7.70 (m, 4H), 7.50 (d, J = 7.6 Hz, 2H), 7.36-7.32 (m, 6H), 7.22-7.04 (m, 4H), 5.20 (s, 2H), 4.0 (* 3.58, s, 2H), 3.82 (* 3.80, s, 3H); ESI-MS: m/z, 469.1 (M+1)+;
(3,5-Dimethyl-Phenyl)-Acetic Acid [4-(4′-Fluoro-Biphenyl-4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 37
White solid; Yield: 80%; M.p.: 130-131oC; IR (KBr): υmax 3673, 3195, 3052, 2922, 2868, 1664, 1603, 1544, 1506, 1464, 1418, 1378, 1340, 1313, 1271, 1228, 1174, 1145, 1074, 1009, 958, 918, 855, 809, 747, 717, 686, 628 cm-1; 1H NMR (500 MHz, DMSO-d6): 11.42 (* 11.40, s, 1H), 8.18 (* 7.84, s, 1H), 7.78-7.64 (m, 4H), 7.58 (d, J = 8.0 Hz, 2H), 7.38-7.26 (m, 3H), 7.18-7.06 (m, 2H), 6.90 (s, 1H), 6.80 (d, J = 8.0 Hz, 2H), 5.20 (s, 2H), 3.98 (* 3.42, s, 2H), 3.82 (* 3.80, s, 3H), 2.22 (s, 3H), 2.0 (s, 3H); ESI-MS: m/z, 497.1 (M+1)+;
(2-Chloro-4-Fluoro-Phenyl)-Acetic Acid [4-(4′-Fluoro-Biphenyl-4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 38
Pale yellow solid; Yield: 88%; M.p.: 109-111oC; IR (KBr): υmax 3209, 3069, 2927, 2867, 1664, 1597, 1567, 1506, 1460, 1385, 1338, 1234, 1170, 1129, 1075, 1002, 963, 920, 855, 808, 731, 684, 643 cm-1; 1H NMR (500 MHz, DMSO-d6): 11.50 (* 11.38, s, 1H), 8.18 (* 7.90, s, 1H), 7.76-7.72 (m, 4H), 7.58 (d, J = 7.8 Hz, 2H), 7.50-7.40 (m, 2H), 7.38-7.24 (m, 3H), 7.20-7.02 (m, 3H), 5.20 (s, 2H), 4.18 (* 3.70, s, 2H), 3.80 (s, 3H); ESI-MS: m/z, 521.1, (M+1)+;
(4-Nitro-Phenyl)-Acetic Acid [4-(4′-Fluoro-Biphenyl-4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 39
Yellow solid; Yield: 92%; M.p.: 109-111oC; IR (KBr): υmax 3465, 3319, 3236, 3065, 2943, 2840, 1667, 1585, 1542, 1503, 1464, 1415, 1351, 1263, 1228, 1132, 1024, 990, 866, 810, 723, 642 cm-1; 1H NMR (500 MHz, DMSO-d6): 11.40 (* 11.20, s, 1H), 8.18 (* 7.98, s, 1H), 8.22 (d, J = 8.2 Hz, 4H), 7.86 (d, J = 8.2 Hz, 4H), 7.64 (d, J = 7.6 Hz, 2H), 7.60 (d, J = 7.6 Hz, 2H), 7.36 (d, J = 8.2 Hz, 1H), 7.20 (d, J = 8.2 Hz, 1H), 7.02 (d, J = 8.2 Hz, 1H), 5.20 (s, 2H), 4.10 (* 3.78, s, 2H), 3.84 (* 3.80, s, 3H);
(2,3-Dihydro-Benzofuran-5-Yl)-Acetic Acid [4-(4′-Fluoro-Biphenyl-4-Ylmethoxy)-3-Methoxy-Benzylidene]-Hydrazide 40
White solid; Yield: 88%; M.p.: 122-123oC; IR (KBr): υmax 3215, 3050, 2967, 2936, 1668, 1601, 1549, 1504, 1455, 1411, 1368, 1346, 1226, 1123, 1031, 991, 943, 817, 683 cm-1; 1H NMR (500 MHz, DMSO-d6): 11.40 (*11.18, s, 1H), 8.18 (*7.82, s, 1H), 7.76-7.66 (m, 4H), 7.58 (d, J = 8.0 Hz, 2H), 7.30-7.26 (m, 3H), 7.18-7.08 (m, 3H), 7.0 (t, J = 8.2 Hz, 1H), 6.62 (dd, J = 6.8, 7.6 Hz, 1H), 5.20 (s, 2H), 4.50 (t, J = 8.2 Hz, 2H), 3.82 (*3.80, s, 3H), 3.86 (*3.40, s, 2H), 3.10 (t, J = 8.2 Hz, 2H); ESI-MS: m/z, 511.1 (M+1)+;
Biology Experimental
Ant-Inflammatory Activity
A standard model system, carrageenan induced inflammatory rat model24 was followed for the experimentation on acute inflammatory conditions. Adult wistar rats weighing between 150-200g were used for the study. Under standard laboratory conditions, rats were maintained (temperature 25 ± 2°C) with normal daily cycle (12/12h). Before the start of experiments, the rats were made to accustom to laboratory condition for 10 days. The study was accordingly permitted by the Institutional Animal Ethical Committee (IAEC) of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals). The animals were starved overnight. Diclofenac sodium (standard drug) at dose of 10 mg/kg and test compounds (31-40, 10 mg/kg i.p), were controlled orally using gastric canula, 30 min before the carrageenan injection in sub plantar region of left hind paw. Paw edema was induced by injecting 0.1 ml of 1% w/v carrageenan suspended in 1% CMC into sub-plantar tissues of the left hind paw of each rat. The degree of paw thickness of all the groups was measured (in millimeters) using a vernier caliper after 1h, 2h and 3h of carrageenan injection.
Conclusions
In conclusion, the present paper describes the synthesis of novel hydrazide-hydrazone derivatives 31-40 embedded with Vanillin and Biphenyl ring moiety and was evaluated for anti-inflammatory activity. Within the series of the synthesized hydrazones, compounds bearing 2-phenylacetohydrazides i.e 39 and 40 exhibited pronounced anti-inflammatory activity when compared to 31, 32, 33, 34 bearing benzohydrazides.
Acknowledgement
One of the authors (ALV) is thankful to Dr. B. Ram, the Director, Green Evolution Laboratories for helpful suggestions and constant encouragement.
References
- Jain, Z. J.; Gide, P. S.; Kankate, R. S. Arabian J. Chem. 2013, doi:10.1016/j.arabjc.2013.07.035
CrossRef - Mojjarad, J. S.; Zamani, Z.; Nazemiyeh, H.; Ghasemi, S.; Asgari, D. Adv. Pharm.Bull, 2011, 1, 9.
- Deep, A.; Jain, S.; Sharma, P.C. Acta Pol. Pharm. Drug Res. 2010, 67, 63.
- Yar, M. S.; Ansari, Z. H. Acta Pol. Pharm. Drug Res 2009, 66, 387.
- Sachan, N.; Thareja, S.; Agarwal, R.; Kadam, S. S.; Kulkarni, V. M. Int. J. Chemtech. Res. 2009, 1, 1625.
- Ruggero, G.; Jones, C. K.; Hemstapat, K.; Nong, Y.; Echemendia, N. G.; Williams, L. C.; Paulis, T. D.; Conn, P. J.; J. Pharmacol.Exp.Ther. 2006, 318, 173.
CrossRef - Deep, A.; Jain, S.; Sharma, P.C.; Verma, P.; Kumar, M.; Dora, C. P.; Acta Pol. Pharm. Drug Res. 2010, 67, 225.
- Madhkar, A.; Kannappan, N.; Deep, A.; Kumar, P.; Kumar, M.; Verma, P. Int. J. Chemtech. Res. 2009, 1, 1376.
- Andrade, H.H.; Santos, J.H.; Gimmler-Luz, M.C.; Correa, M.J.F.; Lehmann, M.; Reguly, M.L. Mutat. Res. 1992, 279, 281.
CrossRef - Ohta, T.; Crit. Rev. 1993, 23, 127.
CrossRef - Akagi, K.; Hirose, M.; Hoshiya, T.; Mizoguchi, Y.; Ito, N.; Shrai, T. Cancer. Lett. 1995, 94, 113.
CrossRef - Tsuda, H.; Uehara, N.; Iwahori, Y.; Asamoto, Y.; Iigo, M.; Nagao, M.; Matsumoto, K.; Ito, M.; Hirono, I. J. Cancer Res. 1994, 85, 1214.
- Gustafson, D.L.; Franz, H.R.; Ueno, A.M.; Smith, J.; Doolittle, D.J.; Waldren, C.A. Mutagenesis. 2007, 15, 207.
CrossRef - Korthou, H.; Verpoorte, R. Flavours and Fragrances-Chemistry. 2007, 9, 203.
CrossRef - Cerrutti, P.; Alzamora, S.M.; Vidales, S.L. J. Food Sci. 1997, 62, 608.
CrossRef - Sardari S.; Nishibe, S.; Daneshtalab, M.; Stud. Nat. Prod. Chem. 2000, 23, 335.
CrossRef - Walton, N.J.; Mayer, M.J.; Narbad, A. Vanillin Phytochem. 2003, 63, 505.
CrossRef - Rollas, S.; Küçükgüzel, S. G. Molecule, 2007, 12, 1910.
CrossRef - Belskaya, N. P.; Dehaen, W.; Bakulev, V. A. Arch. Org. Chem, 2010; 1, 275.
- Xavier, A. J.; Thakur, M.; Marie, J. M. J Chem Pharm Res, 2012, 4, 986.
- Srinivasa Rao Y.; Aminul, Islam.; Nageswar, D.; Hari babu, B.; Asian J Chem, 2015, 27, 3729.
- Kumar Reddy, A.L.V.; Niren, E. Kathale. World Journal of Pharmaceutical Research, 2017, 6 (1), 1255.
- Narang, R.; Narasimhan, B.; Sharma, S. Curr Med Chem, 2012, 19, 569.
CrossRef - Negi, V.J.; Sharma, A. K.; Negi, J. S.; Ra, V. Int J Pharm Chem., 2012; 4, 100.
- Rajasekhar,Narisetty, Chandrasekhar, K.B.; Sandeep, Mohanty.; Balram, B. Letters in Drug Design & Discover. 2013, 10, 620.

This work is licensed under a Creative Commons Attribution 4.0 International License.









