Strength of Material Hap-Borosilicate and their Sintering Behavior
Burmawi1, Novesar Jamarun2, Syukri Arief2, and Gunawarman3
1Department of Mechanical Engineering Bung Hatta University, Padang, Indonesia.
2Department of Chemistry Andalas University, Padang Indonesia.
3Department of Mechanical Engineering, Andalas University, Padang Indonesia.
Corresponding Author E-mail: Novesar@unand.ac.id
DOI : http://dx.doi.org/10.13005/ojc/330242
Article Received on : February 03, 2017
Article Accepted on : March 26, 2017
The high strength of hydroxyapatite is required for biomedical applications .Silicon (Si) and Boron ( Br) are added to get a higher strength material. The mixing process HAp, Si and Br is done by ball milling. This process is followed by the compression with the pressure of 70 kg/cm2 with comparison HAp: Si,Br = 85:15, 90:10 and 95: 5. Finally the mixture is sintered at temperatures of 1000°C. The material is characterized by X-Ray Diffraction (XRD). The hardness of their sample is determined by vickers hardness test. The compressive strength is measured by compression universal test machine. The maximum hardness is obtain for composition of 85:15 that is 47,3 Vickers Hardness Number (VHN) with maximum compressive strength of 3.48 MPa . So it was concluded that Si and Br can be improve the strength of bio composite hydroxyapatite.
KEYWORDS:High Strength; Hydroxyapatite; Biokompatible; Si; Br
Download this article as:| Copy the following to cite this article: Burmawi B, Jamarun N, Arief S, Gunawarman G. Strength of Material Hap-Borosilicate and their Sintering Behaviors. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Burmawi B, Jamarun N, Arief S, Gunawarman G. Strength of Material Hap-Borosilicate and their Sintering Behaviors. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31548 |
Introduction
Hydroxyapatite is a material that can be used as a biomedical material. It can also be used as a material implant1. This material has similarities with human bones2. Hydroxyapatite can be obtained by methods: sol-gel, hydrothermal and precipitation3-6
Hydroxyapatite can be produced from bovine bones7-8. The powder produced bovine bones are extremely brittle or has a very low strength. The addition material to increase the strength is necessary. The added material used should be of bio medical, biocompatible and do not cause bad influence on the human body. Lots of material can be added to hydroxyapatite include Silica (Si) and Boron (Br). The addition of silica at hidroxyapatite can affect the interaction between the ions, electrostatic and in the strengthening of the compound. Silica at high temperatures can serve as a binder9.
The addition of silica 10 effect on the compressive strength. Boron can only be added should not be more than 5% for health, especially for artificial bone applications11. Addition of silica and boron is aiming to create bio composite.
Temperature sintering should be about 1000°C. Since at temperatures above 1000°C can cause structural instability of hydroxyapatite. This process produces tri calcium phosphate12.
The Strength of material for the application of biomaterial is very important. The biomaterials is general have lower compressive strength. The compressive strength and hardness can be enhanced with other techniques. Besides, it is necessary to add materials or other minerals that can improve the strength of the bio composite material13.
In this research, the material used hydroxyapatite from bovine bone has porosity. The material is formed into bio composite. The addition of Silica and Boron elements as a binder material in the formation of HAp-borosilicate bio composite. The Strength of material is determined with the Vickers hardness and compressive strength.
Material and Method
The material used in this research is hydroxyapatite Obtained from bovine bones. Hydroxyapatite mixed with borax acid and silica in the ratio 85: 15: 90: 10 and 95:5. The mixture of materials reviews is constant, HAp: 85, 90 and 95 percent while Boron not more than 5 wt% remainder is silica.
The substitution of Si – Br – HAp was done by using steel ball milling with a speed of 200 rpm for 1 hour, Material that is shaped like a pellet with a diameter of 10 mm followed by formation of compacting pressure 70 Kg/cm2. Sintering conducted at Temperatures of 1000°C for 3 hours. Further tested X-ray diffraction (XRD) and mechanical testing that Vickers hardness test and compression test universal testing machine.
Results and Discussion
XRD
From the figure 1. seen that the compound hydroxyapatite produce from bovine bones.
Meanwhile From XRD peaks seen at figure 2, 3 and 4 the location of hydroxyapatite-borosilicate compound 2θ of 31.7 and 32.2 with Cu Kα . Silica and boron are not too much influence hydroxyapatite compound14. It turned out to silica and boron have subtituted HAp position in the microstructure. cause when compared with a pure hydroxyapatite, the resulting shape of the curve is very similar.
From the graph of XRD seen difference between figure 1 with figure 2, 3 and 4. Figure 1 shows the functional groups of hydroxyapatite compound. In figure 2, 3 and 4 looks change with the advent of a new functional group of hydroxyapatite-Borosilicate.
 |
Figure 1: XRD Pure Hidroxyapatite from bovine bone |
Figure 2, 3 and 4 seen the number of functional groups borosilicate formed. The higher the borosilicate composition, the number of functional groups HAp-Borosilicate is also growing.
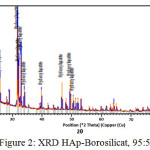 |
Figure 2: XRD HAp-Borosilicat, 95 : 5 |
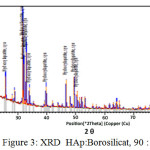 |
Figure 3: XRD HAp:Borosilicat, 90 : 10 |
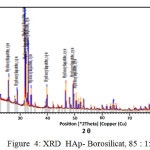 |
Figure 4: XRD HAp- Borosilicat, 85 : 15 |
Ca and P ions can be substituted by ions of silicon and boron. This can be seen in the XRD curve. When compared with the mixing of HAp-Si, Mg15. It is seen a similar pattern. This is due to the interaction between compounds in hydroxyapatite compound. From the results of XRD analysis of the compounds contained impurities but not affecting the hydroxyapatite compounds in general.
The addition silica to the hydroxyapatite is mixed perfectly (Figures 2, 3 and 4 ) when compared with research Bogya et al16. It is because the element boron followers that give effect to the formation of silica glass.
Hardness Test
Hydroxyapatite before added silica and boron have fragile nature. With the addition of silica and boron increased hardness bio composites. From the figure 5. it can be seen that a higher composition of silica and boron additions will improve the material’s hardness value. Looks at the composition of 85:15 has a hardness of 47.66 VHN, the higher the silica composition can increase of the hardness of the material.
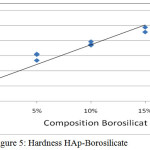 |
Figure 5: Hardness HAp–Borosilicate
|
The sintering temperature of 1000°C to give effect to HAp, Si and Br because phase boron temperatures above 950°C is liquid phase17 and is able to form a bioactive glass18. Crystalline HAp provides a fairly low hardness value range between 0.6 and 1.5 MPa19. The addition of silica 5 % and 10 % on HAp with a sintering temperature of 1000 ° C19 gives the value of the Vickers hardness 111 VHN and 19 VHN but in this research the composition of HAp same turned out to give different hardness, with the addition of the composition of Si and Br, 5 % and 10 % turned out to be an escalation of hardness in the HAp-Borosilicate, this is due to the influence of bioactive borosilicate with a sintering temperature of 1000°C provide vickers hardness values of 28 HV and 36 HV.
In this case an escalation of hardness that is comparable to the increase given borosilicate composition.
Compressive Strength
Silica can serve as a binder to form the HAp-borosilicate bio composites. This affects the compressive strength of the material. From figure 6 the higher the silica and boron composition and generated higher the compressive strengths.
Figure 6 shows the compressive strength values of bio composites HAp-Borosilicate. The maximum value is 3.48 GPa. It is lower in comparison with HAp- Al2O3 with CIG, but has low porosity shortages value with the increasing sintering temperature and decreasing with adding composition20.
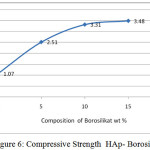 |
Figure 6: Compressive Strength HAp- Borosilicate |
From the graph of an increase in compressive strength follows the curves correspond to the addition of borosilicate
The hardness and compressive strength are visible. Affects hydroxyapatite material composition of compounds that have been substituted by Si and Br. The forming is done by compacting with a pressure of 70 kg/cm2 and at a temperature sintering at 1000 ° C Showed a Considerable effect on strengths of materials significant.
The Compressive strength of materials is increases with the addition of material Silica (Si) and Boron (Br). When compared with pellet hydroxyapatite produce with a pressure of 150 kg / cm2 turned out to be very fragile. HAp-Borosilicate turns giving considerable increase compressive strength significant against21.
Likewise, the value of the compressive strength of the composite HAp, Si, Br turns compositions also Affect the value of the compressive strength of the material. In this case the amount of silica and Boron can Increase the value of the compressive strength of the mixture HAp and Si, Br.
Conclusion
From the above result hydroxyapatite material substitution by Si and Br, however, the number of Si and Br composition can affect the hardness and compressive strength value of the material.
The addition of silica and boron material can improve material hardness and compressive strength bio composite HAp-Borosilicate. Likewise factor mold pressure and temperature sintering .
Acknowledgments
Thanks the author to convey to the Indonesian government, especially Research and Technology Department and Higher Education, which has funded this research.
Refferences
- Nayak, A K.: Bio nano composite, International journal of ChemTech Research,:2010,2,903-907.
- VS Orlovski.:V.S.Komlev and SM Burian.: Biomaterial.: 2002, 1139-1172.
- Sari.T,P:JamarunN,:Syukri,:Azharman Z.:AsregiA.:Orient.J.Chem.,2014,30,1799-1804.
- BingolO.R and Durucan C.: Americ.J.Bio Sci., 2012,4,50-59.
- Eslami H.:Solati-Hashjin M.T.: reza M.: Iran J.Pharm.Sci., 2008,4,127-134.
- Jamarun.N.:Zefri A.:Syukri A.:Tika P.S.: Asregi A.: Elvina S.: Rasayan J.Chem.,2015,8.
- Davids grennspand.: Comparison of A synthetic and Bovine Derived hydroxyapatite Bone Graft Substitute, 2012.
- Singh Anjuvan, Bull Material Science 2012 .,35(6),1031 – 1038.
- Vandiver ,J.: Delphine Dean.: Nelesh P.; Claudia B., Interscience.
- El Yacoubi.A.:A Masit.: M Fathi.B.: Chafic El Idrisi.: K Yamni.: IOSR Journal of Applied Chemistry. 2011, 7, 24-29.
- Deliormanli AM.: Sciverse Science Direct, 2012, 32, 3637- 3646.
- Kim. S.R.: J.H. Lee.: Y.T.Kim.: D.H.Riu.: S.J.Jung.: Y.J.Lee.: S.C.Chung, Elsevier, Biomaterial, 2003, 24, 1389-1396.
- Nakata,K,; Takhasi Kubo,:Chiya Numako,:Takamasa Onoki,:Atsusi Nakahina, Material Transaction, 2009, 50, 1046-1049.
- E.S.Bogya.;R.Barabas.:L.Bizo.:V.R. Dejeu. .Ecer Confrence , 2009.
- B.Cicek,;A.Tucci.;E.Bernardo.;J.Will.;A.R.Boccani. Elsevier, 2014, 6045-6051.
- Roger.F.Brown.;Mohamed.N.Rahaman.;Agatha.B.Dwilewicz.;Wenhai.Huang.;Delbert.E.Day.;Yadong Li.;B.Sonny Bal. Wiley Periodicals Inc, 2008.
- B.Viswanath.;R.Raghavan.;U.Ramamurty and N.Ravishankar, Elsevier, science direct, 2007 361-364.
- B.Bulut.;N.Demirol.;Z.E.Erkmen.;E.S.Kayali, Acta Physica Polonica, 2015, 127.
- Faik Nuzhet Oktar.;Simon Agathopoulos.;L.Sergi Ozyegin.; Oguzhan Gunduz.;Nermin Demirkol.;Yahya Bozkurt.;Serdar Salman, J, Mater Science Med,DOI 10.1007/s 10856-007-3200-9.
- B.Viswanath.:R.Raghavan.:U.Rumamurty and N.Ravishankar. Elsevier.Science Direct Scripta Materialia,2007. 57, 361-364.
- E.Hosseinzadeh.:M.Davarpanah.;N.Hassanzadeh.;S.A.Tavakoh., International Journal of Organ Transpalntation Medicine, 2011.

This work is licensed under a Creative Commons Attribution 4.0 International License.









