Mixed Ligand, Palladium(II) and Platinum(II) Complexes of Tertiary Diphosphines with S-1H Benzo[D] Imidazole-2-Yl Benzothioate
Karwan Omer Ali1, Hikmat Ali Mohammad2, Thomas Gerber3 and Eric Hosten4
1College of Education and Human Sciences, university of Halabja, Halabja.
2Department of Chemistry, college of Education, university of Salahaddin, Erbil.
3Department of Chemistry, Faculty of Science, Nelson Mandela Metropolitan University, Port Elizabeth, 6031, South Africa Chemistry Department.
4Department of Chemistry, Faculty of Science, Nelson Mandela Metropolitan University, Chemistry Department.
Corresponding Author E-mail: karwang1987@gmail.com
DOI : http://dx.doi.org/10.13005/ojc /330205
Article Received on : October 24, 2016
Article Accepted on : January 01, 2017
Palladium(II) and platinum(II) complexes containing the mixed ligands tertiary diphosphines dppm. dppp and dppf with Thioester ligand S-1H benzo[d] imidazole-2-yl benzothioate (HSBIBT) have been prepared by the reaction of PdCl2 and PtCl2 with one equiv of tertiary diphosphines ligands to form [Pd(k2-dppf)Cl2], [Pd(k2-dppp)Cl2] and [Pt(k2-dppmCl)Cl2] complexes and then add the ligand HSBIBT to these complexes to form mixed ligand complexes. The prepared complexes have been characterized by single-crystal X-ray diffraction, elemental analysis, magnetic susceptibility, molar conductance, IR spectral data and UV-Visible. The results suggested that the ligand HSBIBT bonded to the metal through N atom and square planner geometries were assigned for the complexes.
KEYWORDS:Palladium. Platinum; Tertiarydiphosphine; Thioester ligand complexes
Download this article as:| Copy the following to cite this article: Ali K. O, Mohammad H. A, Gerber T, Hosten E. Mixed Ligand, Palladium(II) and Platinum(II) Complexes of Tertiary Diphosphines with S-1H Benzo[D] Imidazole-2-Yl Benzothioate. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Ali K. O, Mohammad H. A, Gerber T, Hosten E. Mixed Ligand, Palladium(II) and Platinum(II) Complexes of Tertiary Diphosphines with S-1H Benzo[D] Imidazole-2-Yl Benzothioate. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=32034 |
Introduction
Benzimidazole is the heterocyclic aromatic organic compound1. This bicyclic ring system consist of benzene ring joined with 4- and 5- situation of imidazole ring, imidazole ring having non adjacent two nitrogen atoms2. 2-Mercaptobenzimidazole derived from benzimidazole with thiol group in the 2-position. It holds extra chemical names such as, o-phenylen thiourea, benzimidazol-2-thion with formula of C7H6N2S3,4. In 2-mercaptobenzimidazole have two N atoms and one S atom and they can independently contribute to its binding to metal5. 2-mercaptobenzimidazole derivatives, one of the most important derivatives of benzimidazole exhibited a wide range of importance biological activities such as antimicrobial6, antihistamine7, neutropic8 and analgesic9, antiprotozoal, antiviral, and anticancer activities10. Metal derivatives of heterocyclic-2-thiones have shown a variety of biochemical application: as antidandruff, antifungal, antibacterial, antibiotic and in shampoos, hair creams, plant diseases and hepatitis11.
Thioesters are important class of organosulfur compounds that play a major role in the construction of pharmaceutical, biological, industrial and natural products. In this respect, thioesters synthesis is one of the most important tasks in organosulfur chemistry. The esterification of thiols is the best and most common strategy for the synthesis of Thioesters12. Diphosphines are a class of chelating ligands that contain two phosphine groups connected by a bridge (also referred to as a backbone). The bridge, for instance, might consist of one or more methylene groups or multiple aromatic rings with heteroatoms attached13. The structure of the backbone and the substituents attached to the phosphorus atoms influence the chemical reactivity of the diphosphine ligand in metal complexes through steric and electronic effects13. Phosphorus ligands typically bind to the metal centers via the lone pair of electrons on the phosphorus14.
Experimental
Materials and Instrumentation
The compounds PtCl2, PdCl2, dppm, dppp, dppf and S-1H benzo[d] imidazole-2-yl benzothioate (HSBIBT) were commercially available and obtained from Yacoo chem. China. The complexes [Pd(k2-dppf)(HSBIBT)Cl]Cl, [Pd(k2-dppp) (HSBIBT)Cl]Cl, [Pt(k2-dppm) (HSBIBT)Cl]Cl were prepared according to the literature.
Elemental CHNS analysis were carried out on a EuroEA 3000 instrument. The FT-IR spectra in range (4000-200 cm-1) were recorded as CsI disc on Shimadzu IRAffinity-1S FTIR Spectrophotometer, and FT-IR spectra in range (4000-400 cm-1) were recorded as KBr disc on Shimadzu, FT-IR spectroscopy Mod IR Affinity-1CE spectrophotometer. UV-Visible spectra were measured using AE-UV1609 (UK) CO., LTD Shimadzu, in the range (200-800) nm. The magnetic susceptibility values of the prepared complexes were carried out at room temperature using Bruker Magnet BM6. Conductivity measurements were made on a conductivity meter type Senz µSiemen tester 4200 (093 cell constant) (UK.). Melting points were measured on Melting Point-MPD-100 Pixel Technology CO., Limited apparatus.
Synthesis of [Pd(k2-dppf)Cl2].CH2Cl2
The solution of dppf (1.6632g, 3mmol) in 15ml CH2Cl2 was added to a solution of PdCl2 (0.5320g, 3mmol) that dissolved in 2 cm3 ethanol and 2 cm3 concentrate HCl. The mixture was reflux for 3 hrs. And then the solvent was evaporate at room temperature to give red precipitate. A small portion of this complex was dissolve in CH2Cl2 and set to slow evaporation at room temperature after a few days, the red colored needle crystals were formed suitable for single-crystal diffraction analysis15. The melting point is 253°C, yield is 2.086gm (95%), formula: [Pd(k2-dppf)Cl2] and M.wt.is 816.58 gm/mole, as shown in Figure 1.
Synthesis of [Pd(k2-dppf)(HSBIBT)Cl]Cl
A solution of ligand (0.0509 g, 0.2 mmol) in CH2Cl2 (15 cm3) was added to a solution of [Pd(k2-dppf)Cl2] (0.1463 g, 0.2 mmol) in CH2Cl2 (15cm3), and the mixture was heated under reflux for 4 hrs and then the Solvent was evaporated at room temperature to give a red solid. Yield= 91 %, m.p= 139-141°C. as shown in Figure 1.
Synthesis of [Pd(k2-dppp)Cl2]
The solution of dppp (1.2373g, 3mmol) in 15ml CH2Cl2 was added to a solution of PdCl2 (0.5320g, 3mmol) that dissolved in 2 cm3 ethanol and 2cm3 concentrate HCl. The mixture was reflux for 3 hrs. And then the solvent was evaporate at room temperature to give pale yellow precipitate. A small portion of this complex was dissolve in CH2Cl2 and set to slow evaporation at room temperature after a one week, the yellow colored needle crystals were formed suitable for single-crystal diffraction analysis15. The Melting point is 277 °C, yield is 1.664gm (94%), formula: [Pd(k2-dppp)Cl2] and M.wt.is 589.72 gm/mole. as shown in Figure 1.
Synthesis of [Pd(k2-dppp)(HSBIBT)Cl]Cl
A solution of ligand (0.0509 g, 0.2 mmol) in CH2Cl2 (15 cm3) was added to a solution of [Pd(k2-dppp)Cl2] (0.1179 g, 0.2 mmol) in CH2Cl2 (15cm3), and the mixture was heated under reflux for 4 hrs and then the Solvent was evaporated at room temperature to give a yellow solid. Yield= 93 %, m.p= 142-144°C. as shown in Figure 1.
Synthesis of [Pt(k2-dppmCl)Cl2]
The solution of dppm (1.1532g, 3mmol) in 15ml CH2Cl2 was added to a solution of PtCl2 (0.7979g, 3mmol) that dissolved in 2 cm3 ethanol and 2cm3 concentrate HCl. The mixture was reflux for 3 hrs. And then the solvent was evaporate at room temperature to give yellow precipitate. A small portion of this complex was dissolve in CH2Cl2 and set to slow evaporation at room temperature after a one week, the yellow colored needle crystals were formed suitable for single-crystal diffraction analysis15. The melting point is ˃ 300°C, yield is 1.756gm (90%), formula: [Pt(k2-dppmCl)Cl2] and M.wt.is 667.43 gm/mole. as shown in Figure 1.
Synthesis of [Pt(k2-dppmCl)(HSBIBT)Cl]Cl
A solution of ligand (0.0509 g, 0.2 mmol) in CH2Cl2 (15 cm3) was added to a solution of [Pt(k2-dppmCl)Cl2] (0.1300 g, 0.2 mmol) in CH2Cl2 (15cm3), and the mixture was heated under reflux for 4 hrs and then the Solvent was evaporated at room temperature to give a yellow solid. Yield= 90 %, m.p= 117-119°C. as shown in Figure 1.
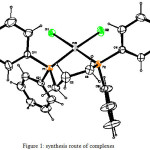 |
Figure 1: synthesis route of complexes |
X-ray Crystallography
Single crystal X-ray crystallography studies were performed at 200 k using a Bruker Kappa Apex II diffractometer with graphite monochromated Mo Kα radiation (λ = 0.71073 °A). For data collection, Apex-II was used while for cell refinement and data reduction, SAINT was used16. The structures were solved by direct methods applying SHELXL-201317, with SHELXLE18 as a graphical interface.
Red crystals of 1, yellow crystals of 3 and yellow crystals of 5 suitable for X-ray crystallographic measurements were obtained by slow evaporation of dichloromethane solutions of the respective complex. Table 4 gives the crystallographic data and collection parameters. All Non-hydrogen atoms were refined with anisotropic displacement parameters. Hydrogen atoms were included in the models by calculating the positions (riding model) and refined with calculated isotropic displacement parameters.
Results and Discussion
The mixed ligand complexes were synthesized by two steps. The first step include the preparation of metal complexes with tertiary diphosphines ligands [MCl2(k2-PPh2(CH2)nPPh2)] where n= 1,3 with [MCl2(k2-dppf)], and M= Pd and Pt. The second step include the synthesis of mixed ligand complexes by addition of S-1H-benzo[d]imidazole-2-yl benzothioate (HSBIBT) ligand to tertiary diphosphines metal complexes. The physical data of complexes are given in Table (1), the suggested molecular formula are supported by IR spectra, UV-Visible sepectra, elemental analysis, magnetic moment and conductivity measurement.
Table 1: physical characteristics and analytical data for metal complexes
| Complex | Color | Yield % | M.P °C |
Elemental analysis, calc. (found) % |
|||
| C | H | N | S | ||||
| [Pd(k2-dppf)(HSBIBT)Cl]Cl | Red | 91 | 142-144 | 58.47(58.47) | 3.84(3.75) | 2.75(2.84) | 3.15(3.24) |
| [Pd(k2-dppp)(HSBIBT)Cl]Cl | Yellow | 93 | 139-141 | 58.45(58.35) | 4.18(4.15) | 3.26(3.32) | 3.46(3.79) |
| [Pt(k2-dppmCl)(HSBIBT)Cl]Cl | Yellow | 90 | 117-119 | 51.46(51.78) | 3.60(3.43) | 2.93(3.09) | 3.44(3.54) |
IR Spectral Studies
The infrared spectrum of free (HSBIBT) ligand is characterized by intense absorption band at 1712 cm-1. This band is assigned to ν(C=O) group19, 4. The stretching frequency of ν(C=N) band which appeared at 1618 cm-1 forfree (HSBIBT) ligand. This band was shifted to lower frequency during complexation due to the coordination of nitrogen atom to the metals20 (Table 2).
The infrared spectra of complexes showed medium intensity bands in the range 513 to 570 cm-1, these bands are attributed to ν(M-N)21. Also showed a bands in the range 370 to 391cm-1, these bands are related to ν(M-Cl)22. Further support for the coordinated phosphines ligands has been provided by the appearance of new bands in the range 285 to 291cm-1, these bands are assigned to ν(M-P)23.
Magnetic Moments
The magnetic moments of complexes [Pd(k2-dppf)(HSBIBT)Cl]Cl (2), [Pd(k2-dppp)(HSBIBT)Cl]Cl (4) and [Pt(k2-dppmCl)(HSBIBT)Cl]Cl (6) are 0.54, 0.6 and 0.44 B.M. respectively (Table 3), suggesting a square planer arrangement around the metal and complexes are diamagnetic24,25.
Electronic Spectral Studies
The UV-Vis spectrum in 10-3 M chloroform (Table 3) shows three absorption bands of Pd(II) complex (2), at 20661 cm-1, 28571 cm-1, and 33783 cm-1 which are assigned to the 1A1g→ 1A2g , 1A1g→ 1Eg and charge transfer transition respectively, these bands are reasonable to square planner geometry26. The UV-visible spectrum of Pd(II) complex(4), gave three spins allowed transitions at 24038 cm-1, 29411 cm-1, and 32679 cm-1 which are assigned to the 1A1g→ 1A2g , 1A1g→ 1Eg and charge transfer transition respectively. The UV-visible spectrum of Pt(II) complex(6), shows three absorption bands at 23923 cm-1, 27777 cm-1, and 32573 cm-1 which are assigned to the 1A1g→ 1A2g , 1A1g→ 1Eg and charge transfer transition respectively, These transitions value are indicated to square planner geometry27.
Conductivity Measurement
The molar conductivity of complexes [Pd(k2-dppf)(HSBIBT)Cl]Cl (2), [Pd(k2-dppp)(HSBIBT)Cl]Cl (4) and [Pt(k2-dppmCl)(HSBIBT)Cl]Cl (6) in dimethyl sulfoxide (DMSO) are 34.2, 41.4 and 36 (cm2.ohm-1. mol-1) (Table 3) suggesting that they are electrolytes complexes28,29.
Table 2: IR frequencies (cm-1) of the compounds
| complex | ν(N-H) | ν(C=O) | ν(C=N) | ν(P-Ph) | ν(P-C) | ν(M-N) | ν(M-P) | ν(M-Cl) |
| HSBIBT | 3151 | 1712 | 1618 | – | – | – | – | – |
| 2 | 3165 | 1712 | 1608 | 1435 | – | 513 | 285 | 370 |
| 4 | 3159 | 1712 | 1612 | 1435 | 513 | 540 | 288 | 383 |
| 6 | 3153 | 1712 | 1608 | 1436 | 509 | 559 | 291 | 391 |
Table 3: Electronic spectra, magnetic moments and molar conductivity of complexes
| complex | λmax(nm)/ Chloroform | assignments | µeff (BM)a | ɅM(Ω-1cm2 mol-1)/ DMSO |
| 2 | 484350296 | 1A1g→ 1A2g1A1g→ 1EgC.T | 0.54 | 34.2 |
| 4 | 416340306 | 1A1g→ 1B1g1A1g→ 1EgC.T | 0.6 | 41.4 |
| 6 | 418360307 | 1A1g→ 1A2g1A1g→ 1EgC.T | 0.44 | 36 |
X-ray Crystal Structures
The [Pd(k2-dppf)Cl2].CH2Cl2 complex was crystallized in a dichorometahne (CH2Cl2) at room temperature. After one week, the red colored needle type crystals had grown from the CH2Cl2 solvent and these crystals were subjected to single-crystal X-ray diffraction study. The molecular structure of [Pd(k2-dppf)Cl2].CH2Cl2 complex is shown in Figure 1. The [Pd(k2-dppf)Cl2].CH2Cl2 complex crystallizes in a monoclinic space group Monoclinic, P21/c. a= 9.7960(3) A, b= 18.1527(7) A, c=19.0713(7) A, α= γ =90°, β=102.536(1). Structural analysis reveals that [Pd(k2-dppf)Cl2].CH2Cl2 complex has a square planer geometry around the metal center, two chlorines and two phosphorus atoms are coordinated with the palladium ion. The dppf behave as bidentate chelating ligand. The crystal determination data and selected bond distances and bond angles for [Pd(k2-dppf)Cl2].CH2Cl2 complex are summarized in Tables 4 and5. The palladium center was found to a distorted square planar geometry, with Cl1-Pd1-P1, Cl2-Pd1-P2, Cl1-Pd1-P2 and Cl2-Pd1-P1 angles of 87.79(2), 83.96(2), 171.50(3), and 177.75(2) respectively [30, 31].
The [Pd(k2-dppp)Cl2] complex was crystallized from a CH2Cl2 solution of the complex at room temperature, after a one week, as yellow colored needle type crystals are formed, these crystals were subjected to single-crystal X-ray diffractometer study. The molecular structure of [Pd(k2-dppp)Cl2] complex is shown in Fig. 2. The structural analysis reveals that [Pd(k2-dppp)Cl2] complex has a distorted square planer geometry around the metal center and that the two phosphorus and two chlorine atoms are coordinated to the palladium ion. The dppm behave as behave as bidentate chelating ligand. Whereas the Pd-P bond distance of 2.2424(4) and 2.2456(4) Ǻ for P(1) and P(2) atoms of the [Pd(k2-dppp)Cl2] complex30,31. The crystal determination data and selected bond distance and bond angles for [Pd(k2- dppp)Cl2] complex is summarized in Tables 4 and 6.
![Figure 2: Molecular Structure of [Pd(k2-dppf)Cl2].CH2Cl2 Complex](http://www.orientjchem.org/wp-content/uploads/2017/04/Vol33No2_Mixe_Karw_fig2-150x150.jpg) |
Figure 2: Molecular Structure of [Pd(k2-dppf)Cl2].CH2Cl2 Complex |
The [Pt(k2-dppmCl)Cl2] complex was crystallized from a CH2Cl2 solution of the complex at room temperature, after a five days, a yellow colored needle type crystals are formed, these crystals were subjected to single-crystal X-ray diffractometer study. The molecular structure of [Pt(k2-dppmCl)Cl2] complex is shown in Fig. 3. The structural analysis reveals that [Pt(k2-dppmCl)Cl2] complex has a square planer geometry around the metal center and that the two phosphorus and two chlorine atoms are coordinated to the platinum ion. The dppm behave as behave as bidentate chelating ligand. Whereas the Pt-P bond length of 2.217(2) and 2.217(2) Ǻ for P(1) and P(2) atoms of the [Pt(k2-dppmCl)Cl2] complex30,31. The crystal determination data and selected bond distances and bond angles for [Pt(k2-dppmCl)Cl2] complex is summarized in Tables 4 and 7.
![Figure 3: Molecular Structure of [Pd(k2-dppp)Cl2] Complex](http://www.orientjchem.org/wp-content/uploads/2017/04/Vol33No2_Mixe_Karw_fig3-150x150.jpg) |
Figure 3: Molecular Structure of [Pd(k2-dppp)Cl2] Complex |
Table 4: Crystallographic Data and structure refinement for [Pd(k2-dppf)Cl2].CH2Cl2, [Pd(k2-dppp)Cl2] and [Pt(k2-dppmCl)Cl2]complexes.
| [Pd(k2-dppf)Cl2].CH2Cl2 | [Pd(k2-dppp)Cl2] | [Pt(k2-dppmCl)Cl2] | |
| Empirical formula | C34H28Cl2FeP2Pd.CH2Cl2 | C27H26Cl2P2Pd | C24.27H21.46Cl2P2Pt(Cl)0.729 (H) 0.54 |
| Formula weight | 816.58 | 589.72 | 667.43 |
| R | 0.03 | 0.02 | 0.03 |
| Temperature | 200 K | 200 K | 200 k |
| Radiation | 0.71073 A | 0.71073 A | 0.71073 A |
| Crstal system | Monoclinic | triclinic | Monoclinic |
| space group | P21/c | P-1 | C2/c |
| a(°A) | 9.7960(3) | 8.4865(3) | 16.2034(5) |
| b(°A) | 18.1527(7) | 10.5484(4) | 7.8274(2) |
| c(°A) | 19.0713(7) | 14.4020(6) | 19.2496(6) |
| Α(deg) | 90 | 88.388(2) | 90 |
| β(deg) | 102.536(1) | 80.064(2) | 98.918(1) |
| γ(deg) | 90 | 73.475(2) | 90 |
| volume | 3310.5(2)A^3 | 1217.16(8) A^3 | 2411.92(12)A^3 |
| F(000) | 1640 | 596 | 4, 1.838 Mg/m^3 |
| Z, Calculated density | 4, 1.638 Mg/m^3 | 2, 1.609 g/m^3 | 6.263 mm^-1 |
| Absorption coefficient | 1.426 mm^-1 | 1.128 mm^-1 | 1288 |
| Crystal size | 0.41 x 0.46 x 0.47 mm | 0.19 x 0.31 x 0.51 mm | 0.04 x 0.20 x 0.21 mm |
| Dataset | -13: 13 ; -20: 24 ; -25: 25 | -11: 11 ; -13: 14 ; -18: 19 | -13:21; -10:10; -25:25 |
| Reflections collected / unique | 60083,8203,[R(int)= 0.018] | 32265, 6016,[R(int)= 0.016] | 16294,3007,[R(int)= 0.030] |
| Observed Data [I > 2.0 sigma(I)] | 7414 | 5668 | 2930 |
| R, wR2, S | R= 0.0284, wR2= 0.0693,S= 1.04 | R= 0.0177, wR2= 0.0451, S= 1.03 | R= 0.0478 wR2= 0.1178 S= 1.30 |
| Min. and Max. Resd. Dens | [-2.08, 1.73] e/Ang^3 | [-0.44,0.44] e/Ang^3 | [-1.71, 4.83] e/Ang^3 |
| Max. and Av | 0.00 and 0.00 | 0.00 and 0.00 | 0.00 and 0.00 |
Table 5: Selected bond distances (in A°) and bond angles (in degree) for [Pd(k2-dppf) Cl2].CH2Cl2
|
Bond distance |
Bond angle |
||
| Pd(1)-Cl(1) | 2.3417(7) | Cl(1)-Pd(1)-P(1) | 87.79(2) |
| Pd(1)-Cl(2) | 2.3577(7) | Cl(1)-Pd(1)-P(2) | 171.50(3) |
| Pd(1)-P(1) | 2.2774(7) | Cl(2)-Pd(1)-P(1) | 177.75(2) |
| Pd(1)-P(2) | 2.2883(7) | Cl(2)-Pd(1)-P(2) | 83.96(2) |
| Fe(1)-C1(1) | 1.996(2) | P(1)-Pd(1)-P(2) | 97.94(2) |
| Fe(1)-C1(2) | 2.028(2) | C1(3)-Fe-C1(4) | 40.16(10) |
| P(1)-C1(1) | 1.802(2) | Pd(1)-P(1)-C(31) | 108.46(7) |
Table 6: Selected bond distances (in A°) and bond angles (in degree) for [Pd(k2-dppp)Cl2]
| Bond distance | Bond angle | ||
| Pd(1)-Cl(1) | 2.3575(4) | Cl(1)-Pd(1)-Cl(2) | 90.54(1) |
| Pd(1)-Cl(2) | 2.3557(4) | Cl(1)-Pd(1)-P(1) | 87.77(1) |
| Pd(1)-P(1) | 2.2424(4) | Cl(1)-Pd(1)-P(2) | 177.80(2) |
| Pd(1)-P(2) | 2.2456(4) | Cl(2)-Pd(1)-P(1) | 172.11(2) |
| P(1)-C(1) | 1.8243(16) | Cl(2)-Pd(1)-P(2) | 91.39(1) |
| P(2)-C(3) | 1.8355(16) | P(1)-Pd(1)-P(2) | 90.47(1) |
| C(3)-H(3)A | 0.9900 | Pd(1)-P(1)-C(1) | 116.42(5) |
Table 7: Selected bond distances (in A°) and bond angles (in degree) for [Pt(k2-dppmCl)Cl2]
| Bond distance | Bond angle | ||
| Pt(1)-Cl(1) | 2.3661(19) | Cl(1)-Pt(1)-P(2) | 98.10(7) |
| Pt(1)-P(2) | 2.217(2) | Cl(1)-Pt(1)-P(1) | 98.10(7) |
| Pt(1)-P(1) | 2.217(2) | Cl(1)-Pt(1)-Cl(1)_a | 90.02(6) |
| Pt(1)-Cl(1)_a | 2.3661(19) | Cl(1)-Pt(1)-P(2)_a | 171.58(7) |
| Pt(1)-P(2)_a | 2.217(2) | Cl(1)_a-Pt(1)-P(2) | 171.58(7) |
| P(1)-C(21) | 1.816(7) | P(2)-Pt(1)-P(2)_a | 73.87(8) |
| P(2)-C(11) | 1.808(8) | Pt(1)-P(1)-C(21) | 117.2(3) |
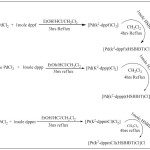 |
Scheme 1
|
![Figure 4: Molecular Structure of [Pt(k2-dppmCl)Cl2] Complex](http://www.orientjchem.org/wp-content/uploads/2017/04/Vol33No2_Mixe_Karw_fig4-150x150.jpg) |
Figure 4: Molecular Structure of [Pt(k2-dppmCl)Cl2] Complex
|
![Figure 5: The Proposed Geometrical Structure of [Pd(k2-dppp)(HSBIBT)Cl]Cl Complex](http://www.orientjchem.org/wp-content/uploads/2017/04/Vol33No2_Mixe_Karw_fig5-150x150.jpg) |
Figure 5: The Proposed Geometrical Structure of [Pd(k2-dppp)(HSBIBT)Cl]Cl Complex Click here to View figure |
![Figure 6: The Proposed Geometrical Structure of [Pt(k2-dppmCl)(HSBIBT)Cl]Cl Complex](http://www.orientjchem.org/wp-content/uploads/2017/04/Vol33No2_Mixe_Karw_fig6-150x150.jpg) |
Figure 6: The Proposed Geometrical Structure of [Pt(k2-dppmCl)(HSBIBT)Cl]Cl Complex Click here to View figure |
Conclusions
In this research we have describe the synthesis and characterization of some Palladium and platinum complexes obtained from the reaction of the Thioester ligand with tertiary diphosphines metal complexes as shown in Figure 1. The manner of bonding and overall structure of complexes were determined through elemental analysis, magnetic susceptibility, molar conductance, IR spectral data and UV-Visible. All synthesized complexes are square planar and diamagnetic.
Acknowledgements
The authors are thankful to the chemistry Department. College of Education-Salahaddin University for their accomplishing our present work and Prof T.I.A. Gerber and Eric. C. Hosten at Nelson Mandela Metropolitan University for their help in obtaining the X-ray crystallographic data.
References
- Walia, R; Hedaitullah, M; Naaz, S.F.; Iqbal, K; Lamba, H. Int. J. Res. Pharm. Chem. 2011, 3, 565-574
- Vikash, K; Deveder, P. Int. Res. J. of Pharm. 2014, 12, 861-875
- Husain, A; Varshney, M; Rashid, M; Mishra, R; Akhter, A. J. of Pharm. Res. 2011, 2, 413-419
- Alias, M; Mahal, D. Int. J. of Sci. and Res. 2015, 7, 1469-1476
- Finšgar, M.Corr. Sci. 2013, 72, 90-98
- Anandara, K; Tiwari, R; Bothara, K; Sunilson, J; Dineshkumar, C; Promwichit, P. Adv. in App.Sci. Res. 2010, 2, 132-138
- Mor, M; Bordi, F; Silva, C; Rivara, S; Zuliani, V; Vacondio, F; Rivara, M; Barocelli, E; Bertoni, S; Ballabeni, V. Bioorg. Medi. Chem. 2004, 4,663-674
- Bakhareva, E; Voronkov, M; Sorokin, M; Lopyrev, V; Seredenin, S; Gaidarov, G. Pharm. Chem. J. 1996, 2,89-91
- Anandara, K; Tiwari, R; Venkateshan, N; Pooshan, G; Promwichit, P. J. Chem and Pharm res. 2010, 3, 230-236
- Thakuria, H; Das, G. Arkivoc. 2008,15, 321-328
- Lobana, T; Sultana, R. J. Chem. Sci. 2012, 6, 1261-1268
- Kazemi, M; Shiri, L. J. Sulf. Chem. 2015, 6,1-11
- Pourshahbaz, M; Irandoust, M; Rafiee, E; Joshaghani, M. Polyhedron. 2009, 3, 609-613
- Cavell, R. Curr. Sci. 2000, 4, 441-452
- Lassahn, P; Lozan, V; Weller, A; Janiak, C. Dalton Trans. 2003, 23, 4437-4450
- APEX; SADABS; SAINT.2010, Bruker AXS Inc., Madison, Wisconsin, USA.
- Sheldrick, G.M. Acta Crystallogr. 2008, A 64, 112-122
- Hubschle, C. B.; Sheldrick, G.M.; Dittrich, B. J Appl Cryst. 2011, 44, 1281-1284
- Enaam, M. R. Diyala J. for Pure Sci. 2013, 1, 97-110
- Pessoa, J; Duarte, M; Gillard, R; Madeira, C; Matias, P; Tomaz, I. J. Chem Soc, Dalton Trans. 1998, 23, 4015-4020
- Amin, O; Al-Hayaly, L; Al-Jibori, S; Al-Allaf, T. Polyhedron. 2004, 11, 2013-2020
- Bu’itrus, N; Hussain, K. Asian J. of Chem. 2003, 4, 1617-1622
- Al-Jibori, S; Al-Jibori, M; Hogarth, G. Inorg. Chim. Acta. 2013, 398, 117-123
- Al-Hayaly, L; Abdullah, B. H.; Al-Dulaimi, A; Al-Jibori, S. A. Oriental J. of Chem. 2008, 2, 381-388
- Edwards. P; Jaouhari, R. Polyhedron. 1989, 1, 25-28
CrossRef - Kumbhare, L; Jain, V; Varghese, B. Inorg. Chim. Acta. 2006, 2, 409-416
- Sutton, D. 1968 McGraw-Hill 192-
- Pastorek, R; Kameníček, J; Krystýnková, P; Šindelář, Z. Acta Universitatis Palackianae Olomucensis. 2000, 39, 63
- Al-Jibori, S; Abdullah, A; Al-Allaf, T. Tran. Metal Chem. 2007, 3,398
- Al-Jibori, S; Al-Jibori, Q; Schmidt, H; Merzweiler, K; Wagner, C; Hogarth, G. Inorg. Chim. Acta. 2013, 402, 69-74
- Steffen, W; Palenik, G. Inorg. Chem. 1976, 10, 2432-2439

This work is licensed under a Creative Commons Attribution 4.0 International License.









