Design, Synthesis, and Anti-Inflammatory Activity of Novel Quinazolines
Said A. El-Feky, Mohd. Imran and Naira Nayeem
Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Northern Border University, Rafha 91911, P.O. Box 840, Kingdom of Saudi Arabia.
Corresponding Author E-mail: drsaid.elfeky@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330217
Article Received on : March 10, 2017
Article Accepted on : March 30, 2017
Several new fluorinated quinazolinone derivatives were prepared and evaluated for in vitro anti-inflammatory activity. The molecular modelling study was performed for compounds 4, 8, 9, 10 and 13. The tested compounds showed strong interactions at the COX-2 binding sites. Compounds 8, 13, 9, and 10 containing triazole, thiadiazole, and oxadiazole rings showed the highest in vitro anti-inflammatory activity and the best binding into the COX-2 binding site.
KEYWORDS:Design; Fluorinated quinazolinones; Molecular modelling study; in vitro anti-inflammatory activity
Download this article as:| Copy the following to cite this article: El-Feky S. A, Imran M, Nayeem N. Design, Synthesis, and Anti-Inflammatory Activity of Novel Quinazolines. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: El-Feky S. A, Imran M, Nayeem N. Design, Synthesis, and Anti-Inflammatory Activity of Novel Quinazolines. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=32139 |
Introduction
Heterocyclic compounds form the basis of many pharmaceuticals, agrochemicals, and veterinary products. Among a wide variety of nitrogen heterocyclic moieties, quinazolinone possess a diverse biological activity profile. The pharmacodynamics versatility of quinazolin-4-one moiety has been documented not only in its synthetic derivatives, but also in several naturally occurring alkaloids isolated from animals, plants, and microorganisms, for example, tryptahthin and rutaecarpine. Also proquazone and fluproquazone are non-acidic anti-inflammatory drugs [1,2]. Celecoxib is a selective COX-2 inhibitory anti-inflammatory drug and has been reported to possess some side effects. Therefore many efforts were performed to develop lead compounds possessing greater efficacy and less side effects than celecoxib [3]. Fluorine atom plays an important role in medicinal chemistry [4,5]. The presence of fluorine atom enhances the pharmacokinetic, the physicochemical properties and the binding to the targeted protein. [6]. Previously, we have synthesized a number of quinoline derivatives bearing a fluorine atom such as compound V (Fig. 1) which exhibited high anti-inflammatory activity in comparison to the celecoxib [7]. On the basis of bioisosterism concept, it was a dream of interest to synthesize compounds 1-17 in the hope that some of them may become a lead compound having potent anti-inflammatory activity [8, 9].
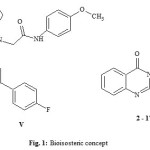 |
Figure 1: Bioisosteric concept Click here to View figure |
Material and Methods
Chemistry
Melting points were obtained by open capillary method using Barnstead 9100 electrothermal melting apparatus. IR spectra (KBr) were generated using Perkin-Elmer spectrometer (ν cm-1). The 1H-NMR spectra were obtained from Varian Gemini-300 spectrophotometer. The 13C-NMR spectra were generated on Brucker 500 MHz spectrophotometer. The mass spectra were obtained with the help of a Perkin-Elmer, Clarus 600 TGC / MS, spectrometer.
2-Mercapto-3-(4-Fluorophenyl)-3H-Quinazolin-4-One
Anthranilic acid (1 mmol) and 4-fluorophenylisothiocyanate (1.5 mmol) in 50 ml ethanol was refluxed for 4 hours. The reaction mixture was cooled at room temperature and the obtained solid was dried and recrystallized from ethanol to give compound 1 in 95% yield. m.p. > 300°C; IR: 1695 (C=O), 2650 (-SH); 1H-NMR (DMSO-D6, δ ppm): 6.95-8.20 (m, 4H, Ar-H of 4-Fluorophenyl), 8.50-8.55 (m, 4H, Ar-H of quinazoline), 12.96 (s, 1H); Mass (m/z): 272 [M+]; Elemental Analysis (C14H9FN2OS): Calcd. C, 61.75; H, 3.33; N, 10.29; Found: C, 61.52; H, 3.47; N, 10.41.
Ethyl-2-[3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin-2-Yl)Thio]Acetate
Compound 1 (1 mmol) and ethyl 2-bromoacetate (1.5 mmol) in 15 ml of acetone containing K2CO3 (1.5 mmol) was stirred for 10 hours at room temperature. The precipitated solid was filtered, and crystallized from ethanol to give compound 2 in 80% yield. m.p. 130-132°C; IR: 1692 and 1735 (C=O); 1H-NMR: 1.56 (t, 3H), 4.02 (s, 2H), 4.30-4.70 (q, 2H), 6.95-8.2 (m, 4H, Ar-H of 4-fluorophenyl), 8.5-8.55 (m, 4H, Ar-H of quinazoline); 13C-NMR: 14.11, 30.81, 60.62, 115.77, 115.69, 119.41, 120.91, 126.66, 126.77, 127.33, 128.33, 130.11, 133.41, 146.99, 159.35, 160.61, 162.91, 169.41; Mass (m/z): 358 (M+); Elemental Analysis (C18H15FN2O3S): Calcd. C, 60.33; H, 4.22; N, 7.82; Found: C, 60.21; H, 4.11; N, 7.86.
2-[3-(4-Fluoropheny)-3,4-Dihydro-4-Oxoquinazolin-2-Yl)Thio]Acetohydrazide
Hydrazine hydrate (2 mmol) was added to compound 2 (1 mmol) in 10 ml absolute ethanol and the reaction mixture was stirred for 12 hours. The solid obtained was filtered and recrystallized from ethanol to give compound 3 in 85% yield. m.p. 170-172°C; IR: 1688 and 1660 (C=O), 3311, 3290, 3245 (N-H); 1H-NMR: 4.22 (s, 2H), 4.31 (s, 2H), 6.95-8.1 (m, 4H, Ar-H of 4-fluorophenyl), 8.50-8.56 (m, 4H, Ar-H of quinazoline), 9.18 (s, 1H); 13C-NMR: 30.44, 115.71, 115.72, 120.81, 126.61, 126.73, 127.32, 128.31, 130.11, 133.42, 146.22, 146.91, 159.33, 160.61, 162.96, 170.31; Mass (m/z): 344 (M+); ; Elemental Analysis (C16H13FN4O2S): Calcd. C, 55.81; H, 3.81; N, 16.27; Found: C, 55.66; H, 3.86; N, 16.31.
(E)-N-(4-Fluorobenzylidene)-2-[3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin-2-Yl)Thio]Acetohydrazide
Compound 3 (1.5 mmol) and p-fluorobenzaldehyde (1.5 mmol) in 15 ml of absolute ethanol was stirred for 1 hour. The obtained solid was filtered and crystallized from ethanol to give compound 4 in 81% yield. m.p. 182-184°C; IR: 1687 and 1655 (C=O), 3315 (-NH-), 1H-NMR: 4.18 (s, 2H), 6.96-8.10 (m, 4H, Ar-H of 4-fluorophenyl), 8.50-8.55 (m, 4H, Ar-H of quinazoline), 10.95 (s, 0.5H), 11.05 (s, 0.5H); 13C-NMR: 30.77, 115.61, 115.61, 116.71 (2C), 120.81, 126.63, 126.71, 127.33, 128.33, 129.38, 130.16, 130.16, 130.81, 138.11, 138.82, 144.11, 146.11, 148.11, 160.61, 162.93, 165.22, 171.11; Mass (m/z): 450 (M+); Elemental Analysis (C23H16F2N4O2S): Calcd. C, 61.33; H, 3.58; N, 12.44; Found: C, 61.21; H, 3.55; N, 12.51.
Synthesis of Compounds 5a-c
To compound 1 (2 mmol) in 15 ml acetone, the respective 2-chloro-N-(substitutedphenyl)anilide (2.1 mmol) and anhydrous K2CO3 (3 mmol) was added and refluxed with stirring for 1 hour. The precipitated solid was filtered, washed with water, and recrystallized from ethanol.
2-[3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin-2-Yl)Thio]Acetamide
Yield 85%; m.p. 177-179°C; IR: 1682 and 1651 (C=O), 3180 (N-H); 1H-NMR: 4.19 (s, 2H), 6.91-8.10 (m, 4H, Ar-H of 4-fluorophenyl), 8.50-8.55 (m, 4H, Ar-H of quinazoline), 8.54 (s, 1H); 13C-NMR: 30.92, 115.71, 115.72, 120.81, 126.62, 126.63, 126.71, 127.33, 128.33, 130.11, 133.41, 146.91, 159.31, 160.63, 162.92, 170.81; Mass (m/z): 329 (M+); Elemental Analysis (C16H12FN3O2S): Calcd. C, 58.35; H, 3.67; N, 12.76; Found: C, 58.22; H, 3.55; N, 12.55.
2-[3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin-2-Yl)Thio]-N-Phenylacetamide
Yield 95%; m.p. 162-164°C; IR: 1683 and 1655 (C=O), 3183 (N-H); 1H-NMR: 4.22 (s, 2H), 6.92-8.30 (m, 8H, Ar-H), 8.50-8.55 (m, 4H, Ar-H of quinazoline), 8.57 (s, 1H); 13C-NMR: 28.33, 115.71, 115.72, 121.61, 121.62, 126.61, 126.72, 127.31, 128.11, 128.81, 128.33, 128.91, 130.32 (2C), 132.44, 133.41, 139.51, 146.92, 159.31, 160.61, 162.91, 168.22; Mass (m/z): 405 (M+); Elemental Analysis (C22H16FN3O2S): Calcd. C, 65.17; H, 3.98; N, 10.36; Found: C, 65.22; H, 3.65; N, 10.22.
2-[3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin-2-Yl)Thio]-N-(4 Fluorophenyl)Acetamide
Yield 85%; m.p. 152-154°C; IR: 1682 and 1654 (C=O), 3182 (N-H); 1H-NMR: 4.22 (s, 2H), 6.93-8.33 (m, 8H, Ar-H), 8.50-8.56 (m, 4H, Ar-H of quinazoline), 8.58 (s, 1H); 13C-NMR: 29.22, 81.21, 110, 11, 115.71, 115.72, 120.81, 125.71, 126.61, 126.71, 127.31, 128.33, 130.11 (2C), 131.33, 133.41, 137.51, 137.51, 146.91, 159.31, 160.61, 162.91, 167.33; Mass (m/z): 423 (M+); Elemental Analysis (C22H15F2N3O2S): Calcd. C, 62.40; H, 3.57; N, 9.92; Found: C, 62.33; H, 3.81; N, 9.88.
2-(4-Fluorophenacyl)Thio]-3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin
Compound 1 (1 mmol) in 20 ml acetone, 4-fluorophenacyl bromide (1.1 mmol) and K2CO3 (2 mmol) was heated under reflux for 5 hours. Ice cold water was added to the reaction mixture and the solid obtained was filtered and recrystallized from ethanol to give compound 6 in 71% yield. m.p. 157-159°C; IR: 1682 and 1654 (C=O); 1H-NMR: 3.25 (s, 1H), 4.22 (s, 2H), 6.96-8.3 (m, 8H, Ar-H), 8.50-8.55 (m, 4H, Ar-H of quinazoline); 13C-NMR: 35.81, 115.41, 115.42, 115.71, 115.72, 120.81, 126.61, 126.71, 127.31, 128.31, 130.11 (2C), 131.0, 131.11 (2C), 133.41, 146.91, 159.31, 160.61, 162.91, 167.33, 194.11; Mass (m/z): 408 (M+); Elemental Analysis (C22H14F2N2O5S): Calcd. C, 64.70; H, 3.46; N, 6.86; Found: C, 64.66; H, 3.55; N, 7.11.
2-[2-[3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin-2-Yl]Thio]Acetyl-N-(4-Fluorophenylhydrazine)-1-Carbothioamide
Compound 3 (2 mmol) in 20 ml absolute ethanol was refluxed with 4-fluorophenylisothiocyanate (2.1 mmol) for 1 hour. The obtained solid was filtered and recrystallized from ethanol to give compound 7 in 83% yield. m.p. 171-173°C; IR: 3384, 3187 (2 N-H), 1676, 1650 (C=O) and 1350 (C=S); 1H-NMR: 4.01 (s, 2H), 6.94-8.20 (m, 8H, Ar-H), 8.51-8.56 (m, 4H, Ar-H of quinazoline), 10.33 (s, 1H), 9.24 (s, 1H), 8.61 (s, 1H); 13C-NMR: 31.11, 115.71, 115.72, 115.81, 115.82, 120.81, 126.61, 126.71, 127.32, 128.31, 128.32, 130.11, 131.11 (2C), 131.22, 133.41, 146.91, 159.33, 160.62, 162.91, 163.33, 170.33, 181.11; Mass (m/z): 497 (M+); Elemental Analysis (C23H17F2N5O2S2): Calcd. C, 55.52; H, 3.44; N, 14.08; Found: C, 55.66; H, 3.35; N, 14.11.
2-([(4-Fluorophenyl-5-Mercapto-4H-1,2,4-Triazol-3-Yl)Methyl]Thio)-3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin
Compound 7 (2 mmol) was refluxed with aqueous KOH (3 mmol) for 2 hours. The clear solution was neutralized with 10% HCl and the obtained solid was filtered, washed with water and recrystallized from ethanol to give compound 8 in 81% yield. m.p. 221-223°C; IR: 1687 (C=O); 1H-NMR: 4.47 (s, 2H), 6.93-8.31 (m, 8H, Ar-H), 8.51-8.53 (m, 4H, Ar-H of quinazoline), 13.89 (s, 1H); 13C-NMR: 29.11, 115.71 (2C), 115.81 (2C), 120.81, 126.61, 127.33, 128.31, 129.51, 130.11 (2C), 133.41, 135.11, 135.12, 146.91, 157.11, 159.33, 160.63, 162.92, 163.31, 166.71; Mass (m/z): 479 (M+); Elemental Analysis (C23H15F2N5OS2): Calcd. C, 57.61; H, 3.15; N, 14.61; Found: C, 57.55; H, 3.16; N, 14.21.
2-([(5-(4-Fluorophenyl)Amino)1,3,4-Thiadiazole-2-Yl)Methyl]Thio)-3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin
Compound 7 (2 mmol) and concentrated H2SO4 (5ml) was stirred at room temperature for 12 hours. The reaction mixture was neutralized with KHCO3 and the obtained solid was crystallized to give compound 9 74% yield. m.p. 228-230°C; IR: 3182 (N-H) and 1683 (C=O); 1H-NMR: 4.67 (s, 2H), 6.92-8.23 (m, 8H, Ar-H), 8.52-8.56 (m, 4H, Ar-H of quinazoline), 9.82 (s, 1H, NH); 13C-NMR: 24.41, 115.71, 115.72, 116.31, 116.33, 120.62, 120.82, 126.62, 126.72, 127.34, 128.33, 128.36, 130.11, 133.41, 138.11, 146.91, 152.72, 157.31, 159.32, 159.33, 160.61, 162.92, 168.01; Mass (m/z): 479 (M+); Elemental Analysis (C23H15F2N5OS2): Calcd. C, 57.61; H, 3.15; N, 14.61; Found: C, 57.55; H, 3.33; N, 14.75.
2-([(5-(4-Fluorophenyl)Amino)-1,3,4-Oxadiazol-2-Yl)Methyl]Thio-3-(4-Fluorophenyl)-3,4-Dihydro-4-Oxoquinazolin
To compound 7 (1 mmol) in toluene (20 ml), DCCD (1.5 mmol) was added and the reaction mixture was refluxed for 6 hours. On cooling the obtained solid was filtered and recrystallized from ethanol to give compound 10 in 63% yield. m.p. 271-273°C; IR: 3182 (N-H) and 1686 (C=O); 1H-NMR: 4.62 (s, 2H), 4.68 (s, 1H), 6.91-8.28 (m, 8H, Ar-H), 8.52-8.56 (m, 4H, Ar-H of quinazoline), 9.88 (s, 1H); 13C-NMR: 24.41, 115.71, 115.72, 116.32, 116.33, 116.34, 120.61, 120.62, 120.64, 120.82, 126.71, 127.33, 128.34, 130.11, 130.13, 134.51, 146.92, 157.33, 159.32, 160.61, 162.92, 163.24, 169.33; Mass (m/z): 463 (M+); Elemental Analysis (C23H15F2N5O2S): Calcd. C, 69.61; H, 3.26; N, 15.11; Found: C, 69.55; H, 3.11; N, 15.22.
Ethyl 2-(3-(4-Fluorophenyl)-4-Oxo-3,4-Dihydroquinazolin-2-Yl)Thio)Propanoate
Compound 1 (1 mmol) was dissolved in 15 ml of absolute ethanol and KOH (1.5 mmol). Ethyl ethyl-2-bromopropanoate (1.5 mmol) was added dropwise to the reaction mixture and refluxed for 6 hours. The reaction mixture was poured on ice cold water and the precipitated solid was filtered, and recrystallized from ethanol to give compound 11 in 75% yield. m.p. 190-192°C; IR: 1733 (C=O, ester), 1687 (C=O, quinazolinone); 1H-NMR: 0.82 (t, 3H), 1.19 (d, 3H), 2.73 (q, 1H), 3.32 (q, 2H), 7.20-8.20 (m, 8H, Ar-H); 13C-NMR: 14.11, 18.52, 37.11, 60.92, 115.72, 120.81, 126.61, 126.72, 128.72, 128.33, 130.11, 130.12, 133.41, 146.92, 155.74, 159.32, 160.62, 162.92, 170.82; Mass (m/z): 372 (M+); Elemental Analysis (C19H17FN2O3S): Calcd. C, 61.28; H, 4.60; N, 7.52; Found: C, 61.33; H, 4.77; N, 8.11.
2-(3-(4-Fluorophenyl)-4-Oxo-3,4-Dihydroquinazolin-2-Yl)Thio)Propanoyl Semicarbazide
Compound 11 (1 mmol), semicarbazide (1.1 mmol) and anhydrous sodium acetate (2 mmol) was refluxed in DMF (15 ml) for 20 hours. Ice cold water was added to the reaction mixture and the precipitated solid was filtered and recrystallized from ethanol to give compound 12 in 80% yield. m.p. 220-222°C; IR: 3447 (NH2), 3218 (N-H), 1683, 1681 and 1650 (C=O); 1H-NMR: 1.56 (d, 3H), 2.83 (q, 1H), 6.05 (s, 2H), 7.41-8.31 (m, 8H, Ar-H), 9.72 (s, 1H, NH); 13C-NMR: 20.42, 39.12, 115.77, 115.76, 120.81, 126.63, 126.72, 127.34, 128.35, 130.11, 130.16, 133.44, 146.91, 157.41, 159.33, 160.62, 162.93, 174.82; Mass (m/z): 401 (M+); Elemental Analysis (C18H16FN5O3S): Calcd. C, 53.86; H, 4.02; N, 17.45; Found: C, 53.77; H, 4.11; N, 17.66.
3-[1-(3-(4-Fluorophenyl)-4-Oxo-3,4-Dihydroquinazolin-2-Yl-Thio)Ethyl]-4H-1,2,4-Triazol-5-Ol
Compound 12 (1 mmol) and 20% aqueous KOH (25 ml) was refluxed for 12 hours. The mixture was cooled, poured into ice cold water, stirred and neutralized by gradual addition of (1:1) hydrochloric acid. The precipitate was filtered, washed with H2O and recrystallized from ethanol to give compound 13 in 90% yield. m.p. > 300°C; IR: 3444 (O-H), 3377 (N-H), 1685 (C=O), 1639 (C=N); 1H-NMR: 1.58 (d, 3H), 2.83 (q, 1H), 5.1 (s, 1H), 7.41-8.32 (m, 8H, Ar-H), 9.81 (s, 1H); 13C-NMR: 17.91, 33.81, 115.72, 115.75, 120.81, 126.61, 127.32, 128.33, 128.73, 131.11, 131.12, 133.44, 148.92, 152.11, 159.33, 159.34, 160.61, 162.93; Mass (m/z): 383 (M+); Elemental Analysis (C18H14FN5O2S): Calcd. C, 56.39; H, 3.68; N, 18.27; Found: C, 56.29; H, 3.55; N, 18.11.
2-(1-β-cyanoethyl-4-(4-fluorophenyl)-5-thioxo-∆2-s-triazolin-3-ylmethyl)-3-(4-fluorophenyl) -3,4-dihydro-4-oxo-quinazolin
The solution of compound 8 (1 mmol) in water (5 ml) and pyridine (20 ml) was treated with acrylonitrile (1 ml). The mixture was refluxed for 4 hours and the volume was reduced to half. The solid was filtered, washed with water and recrystallized from ethanol to give compound 14 in 50% yield. m.p. 172-174°C; IR: 1682 (C=O), 2230 (CN); 1H-NMR: 1.16 (t, 2H), 3.98 (t, 2H), 4.20 (s, 2H), 6.92-8.23 (m, 8H, Ar-H), 8.52-8.56 (m, 4H, Ar-H of quinazoline); 13C-NMR: 29.91, 115.71, 115.81, 120.81, 125.62, 125.71, 126.61, 126.71, 127.33, 127.34, 127.38, 128.55, 128.56, 130.11, 130.13, 130.14, 133.42, 135.01, 135.03, 146.91, 157.81, 159.22, 160.61, 162.9, 167.61, 190.33; Mass (m/z): 532 (M+); Elemental Analysis (C26H18F2N6OS2): Calcd. C, 58.64; H, 3.41; N, 15.78; Found: C, 58.55; H, 3.22; N, 15.66.
2-(4-(4-fluorophenyl)-5-methylthio-s-triazol-3-ylmethyl)-3-(4-fluorophenyl)-3,4-dihydro-4-oxoquinazoline
To a mixture of 8 (1 mmol) and fused sodium acetate (2 mmol), methyl iodide (1 mmol) was added. The mixture was heated for 2 hours, cooled to RT and poured on crushed ice, then scratched with a glass rod and kept overnight in a refrigerator. The white solid was filtered and recrystallized from ethanol to give compound 15 in 65% yield. m.p. 185-187°C; IR: 1683 (C=O); 1H-NMR: 2.43 (s, 3H), 4.28 (s, 2H), 6.92-8.23 (m, 8H, Ar-H), 8.52-8.56 (m, 4H, Ar-H of quinazoline); 13C-NMR: 14.81, 21.42, 113.42, 115.51, 115.52, 115.72, 115.73, 120.82, 125.52, 126.26, 126.71, 127.33, 128.35, 128.41, 130.11, 130.14, 140.11, 141.42, 146.91, 147.33, 153.01, 159.32, 162.91, 162.94; Mass (m/z): 493 (M+); Elemental Analysis (C24H17F2N5OS2): Calcd. C, 58.41; H, 3.47; N, 14.19; Found: C, 58.22; H, 3.55; N, 14.20.
2-(4-(4-fluorophenyl)-1-morpholinomethyl)-5-thioxo-∆2-s-triazolin-3-ylmethyl)-4-(4-fluorophenyl)-3,4-dihydro-4-oxoquinazolin
An ethanolic solution of 8 was stirred with 37% formaldehyde solution (1 ml) and ethanolic solution of morpholine (9 mmol) for 2 hours at RT. The mixture was kept in a refrigerator overnight and the precipitated solid was filtered, washed with cold ethanol and recrystallized from ethanol to give compound 16 in 90% yield. m.p. 215-217 oC; IR: 1683 (C=O); 1H-NMR: 2.76 (m, 4H), 3.76 (m, 4H), 4.21 (s, 2H), 6.92-8.23 (m, 8H, Ar-H), 8.52-8.56 (m, 4H, Ar-H of quinazoline); 13C-NMR: 2.31, 2.32, 2.38, 2.39, 19.21, 24.42, 36.92, 59.21, 104.32, 105.81, 113.42, 115.11, 115.72, 115.73, 126.33, 126.62, 127.33, 128.32, 128.71, 130.11, 130.13, 133.41, 141.42, 146.92, 151.01, 159.32, 160.41, 162.43; Mass (m/z): 578 (M+); Elemental Analysis (C28H24F2N6O2S2): Calcd. C, 58.18; H, 4.18; N, 14.52; Found: C, 58.17; H, 4.11; N, 14.57.
2-(4-(4-Fluorophenyl)-3-methyl-5-thioxo-∆2-s-triazolin-3-ylmethyl)-3-(4-fluorophenyl)-3,4-dihydro-4-oxoquinazoline
The solution of 15 (5 mmol) and methyl iodide (9 mmol) in DMF (20 ml) was heated for one hour. The mixture was poured into ice cold water and the precipitate was recrystallized from ethanol to give compound 17 in 60% yield. m.p. 196-198°C; IR: 1682 (C=O), 1140 and 1470 (C=S); 1H-NMR: 2.55 (s, 3H), 4.29 (s, 2H), 6.92-8.29 (m, 8H, Ar-H), 8.51-8.57 (m, 4H, Ar-H of quinazoline); Elemental Analysis (C24H17F2N5OS2): Calcd. C, 58.41; H, 3.47; N, 14.19; Found: C, 58.41; H, 3.47; N, 14.11.
In Vitro Anti-Inflammatory Activity
It was carried out by the colorimetric COX (ovine) inhibitor screening assay adopting the reported procedure [10-12].
Docking Methodology
Molecular modelling was performed as provided in our previous report [8].
Results and Discussion
Chemistry
Compound 1 was obtained via the reaction of anthranilic acid and 4-fluorophenyl isothiocyante (Scheme 1). The IR spectra of compound 1 revealed a signal at 1690 cm-1 corresponding to C=O of quinazolinone 1. Reaction of compound 1 with ethyl bromoacetate gave the corresponding ester 2 which on reaction with hydrazine hydrate in ethanol produced the corresponding hydrazide 3. Reaction of compound 3 with 4-fluorobenzaldehyde produced the corresponding hydrazone derivative 4. On the other hand, treatment of 1 with 2-chloro-N-(substitutedphenyl)anilides gave the corresponding acetamide derivatives 5a-c. Moreover, reaction of 1 with 4-fluorophenacyl bromide gave compound 6 (Scheme 1).
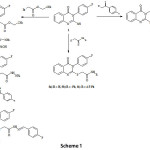 |
Scheme 1 Click here to View scheme |
Scheme 2 was achieved by reaction of compound 3 with 4-fluorophenylisothiocyanate to produce the carbothioamide derivative 7 which was used as starting compound for the synthesis of the corresponding triazole, thiadiazole and oxadiazole derivatives. Compound 7 was reacted with aqueous KOH under refluxed to give the corresponding triazole derivative 8. Similarly, when compound 7 was stirred with concentrated sulfuric acid, it gave the corresponding 1,3,4-thiadiazole derivative 9. Finally, the compound 7 was cyclodesulfurised by hrating under reflux with DCCD in toluene to give the oxadiazole derivative 10.
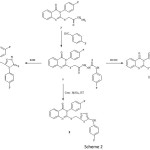 |
Scheme 2 Click here to View scheme |
The compound 11 was prepared by the reaction of the compound 1 with ethyl-2-bromopropanoate (Scheme 3) to give the ester 11 which was converted to the corresponding semicarbazide 12 by the reaction with semicarbazide. The corresponding hydroxyl triazole derivative 13 was obtained by cyclocondenzation of 12 with aqueous NaOH.
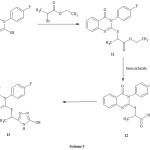 |
Scheme 3 Click here to View scheme |
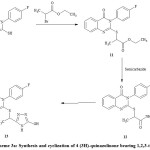 |
Scheme 3a: Synthesis and cyclization of 4 (3H)-quinazolinone bearing 1,2,3-triazole ring |
The triazole derivative 8 was subjected to cyanomethylation using acrylonitrile or aminomethylation using Mannich reaction conditions to afford the products 14 and 16, respectively. Methylation of 8 in acetone in the presence of sodium acetate resulted in the kinetically controlled reaction product 15 as proved by the conversion of 15 to the thermodynamically controlled reaction product 16 upon heating in dimethylformamide in the presence of methyl iodide, as what have been reported by Molina and Alajarin [13]. Structure elucidation of the N-substitution was confirmed by elemental analysis as well as spectral data.
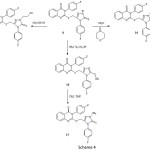 |
Scheme 4 Click here to View scheme |
In Vitro COX Inhibition Assay
The highly scored docked derivatives 4, 8, 9, 10, and 13 were evaluated using an in vitro COX-1 / COX-2 inhibition assay (Table 1).
Table 1: Values for in vitro COX-1 / COX-2 enzyme inhibition by the designed compounds
|
Compound No. |
COX-1 |
COX-2 |
SI |
|
4 |
> 100 |
0.33 |
> 303.0 |
|
8 |
> 100 |
0.83 |
> 125.0 |
|
9 |
> 100 |
0.42 |
> 250 |
|
10 |
> 100 |
0.73 |
> 142.9 |
|
13 |
> 100 |
0.31 |
> 333.3 |
Molecular Modelling Studies
These were performed using MOE 2007.09 program [14]. The crystallographic enzyme ligand complex with SC-558 was obtained from the RCSB protein data bank (PDB entry ICX2) [15, 16].
Table 2: Molecular modeling data for compounds 4, 8, 9, 10, 13 and Celecoxib during docking in COX-2 (PDB ID: 1CX2) active site
|
Compound |
COX-2 |
|||||
|
Affinity Kcal/mol |
Distance (in Ao) from main residue |
Functional group |
Interaction |
2d caption |
||
|
4 |
0.9 -5.3 -1.0 |
3.01 3.13 3.88 |
Glu524 Glu524 |
-S- -Ph-ring |
H-donor H-donor pi-cation |
Fig. 2
|
|
8 |
-0.6 -0.1 -1.1 -2.2 -1.3 |
4.03 3.31 2.94 3.60 |
Val349 Ser353 Arg513 Arg513 |
-S- -S- =N- =S =S |
H-donor H-donor H-acceptor H-acceptor H-acceptor |
Fig. 3
|
|
9 |
-1.4 -0.6 |
3.70 4.36 |
Lys83 Arg513 |
-S- -Ph-ring |
H-acceptor pi-cation |
Fig. 4 |
|
10 |
-5.1 -11.3 -0.7 |
2.82 3.06 4.08 |
Glu524 Arg513 |
-NH- =N- -Ph-ring |
H-donor |
Fig. 5 |
|
13 |
-1.2 -1.8 |
3.13 3.14 |
Arg120 Arg120 |
=N- =N- |
H-acceptor H-acceptor |
Fig. 6 |
|
Celecoxib |
-6.5 |
2.91
|
Arg513 |
-SO2 -SO2 |
H-acceptor |
Fig. 7 |
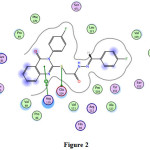 |
Figure 2 Click here to View figure |
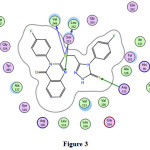 |
Figure 3 |
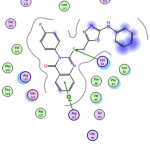 |
Figure 4 Click here to View Figure |
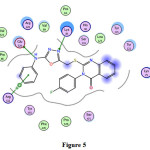 |
Figure 5 Click here to View figure |
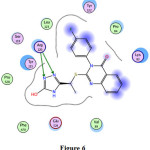 |
Figure 6 Click here to View figure |
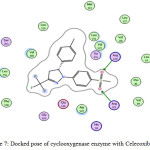 |
Figure 7: Docked pose of cyclooxygenase enzyme with Celecoxib (2D). |
Conclusion
In the present study, novel fluorinated quinazolinones having triazole, thiadiazole, and oxadiazole rings were synthesized and evaluated for their in vitro anti-inflammatory activity. Molecular modelling studies were performed to examine the selectivity on cyclooxygenase 2 enzyme. Compounds 4, 8, 9, 10, and 13 showed interesting anti-inflammatory activity and a good binding with the COX-2 enzyme. Therefore, these compounds may represent lead compounds for developing anti-inflammatory agents with high binding affinity with the receptor and no side effects. Also, fluorine will very likely continue to contribute in drug design and discovery by playing multifaceted [17, 18] roles in enhancing future medical advances.
Acknowledgements
The authors gratefully acknowledge the approval and support of this research study by the grant no. PHM-2016-1-6-F-5682 K.S.A. at Northern Border University, Arar from the Deanship of Scientific Research.
References
- Lee E.S., Kim S.I, Lee S.H., Jeong T.C., Moon T.C., Chang H.W., Jahng Y.D., Bull. Korean Chem. Soc. 2005, 26(12), 1975-1980.
- Jahng Y., Arch. Pharm. Res. 2013, 36(5), 517-535.
- Abdel-Aziz A.A., Abou-Zeid L.A., ElTahir K.E., Mohamed M.A., Abu El-Enin M.A., El-Azab A.S. Bioorg. Med. Chem. 2016, 24(16), 3818-3828.
- Mahin R., Ali D. J. Fluorine Chem. 2017, 193, 89-97.
- Wei W., Yuan Z., Hao P., Hong-Wu H., Xing-Tao L. J. Fluorine Chem. 2017, 193, 8-16.
- Shah P., Westwell A.D. J. Enzyme Inhib. Med. Chem. 2007, 22(5), 527-540.
- Said A.E., Hamdy K.T., Mustafa T.U. J. Fluorine Chem. 2014, 161, 87-94.
- Said A.H.E., Zakaria K.A.E., Nermine A.O., Jasmine L., Mohamed A.K., Hamdy K.T. Bioorg. Chem. 2015, 58, 104-116.
- Said A.E., Hamdy K.T., Mahmoud M.E.M. Orient. J. Chem. 2015, 31(2), 709-718.
- El-Sayed M.A., Abdel-Aziz N.I., Abdel-Aziz A.A., El-Azab A.S., Asiri Y.A., Eltahir K.E. Bioorg. Med. Chem. 2011, 19(11), 3416-3424.
- Alanazi A.M., El-Azab A.S., Al-Suwaidan I.A., ElTahir K.E., Asiri Y.A., Abdel-Aziz N.I., Abdel-Aziz A.A. Eur. J. Med. Chem. 2015, 92, 115-123.
- Uddin M.J., Rao P.N., Knaus E.E.. Bioorg. Med. Chem. 2004, 12(22), 5929-5940.
- Molina P., Alajarin M. Synthesis 1983, 5, 414-415.
- https://www.chemcomp.com/MOE-Molecular_Operating_Environment.htm
- Kurumbail R.G., Stevens A.M., Gierse J.K., McDonald J.J., Stegeman R.A., Pak J.Y., Gildehaus D., Miyashiro J.M., Penning T.D., Seibert K., Isakson P.C., Stallings W.C. Nature. 1996, 384(6610), 644-648.
- Xu S., Hermanson D.J., Banerjee S., Ghebreselasie K., Clayton G.M., Garavito R.M., Marnett L.J. J. Biol. Chem. 2014, 289(10), 6799-6808.
- Iwao O. J. Org. Chem. 2013, 78(13), 6358-6383.
- Fyaz M.D. J. Fluorine Chem. 2002, 118, 1-2, 27-33.

This work is licensed under a Creative Commons Attribution 4.0 International License.









