Biosynthesis and Characterization of Zinc Sulphide Nanoparticles using Leaf Extracts of Tridaxprocumbens
M. Sathishkumar1,2, M. Saroja1, M. Venkatachalam1, G.Parthasarathy1 and A. T. Rajamanickam2
1Department of Electronics, Erode Arts and Science College, Erode, Tamilnadu -638009.
2Department of electronics and communication systems, Nehru Arts and Science College, Coimbatore, Tamilnadu -641105.
Corresponding Author E-mail: mskeasc@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330240
Article Received on : March 06, 2017
Article Accepted on : March 09, 2017
In this paper, we discussed biosynthesized and characterization of zinc sulphide nanoparticles using tridaxprocumbens leaf extract by green synthesis method. This study focuses on the production of ZnS nanoparticles using tridaxprocumbens plant of methanol leaf extracts and analysis the antimicrobial activity. The formation of zinc sulphide nanoparticleswas characterized by using numerous analytical techniques such as Scanning electron microscopy (SEM), Fourier transform infrared spectroscopy(FT-IR), X-ray powder diffraction (XRD) and UV-Visible spectroscopy (UV-Vis). The results showed that the leaf extract for the synthesis zinc sulphide nanoparticles to have the ability to inhibit the growth of various pathogenic microorganisms. The synthesized zinc sulphide nanoparticles can be used for various applications due to its ecofriendly, non-toxic and compatibility for pharmaceutical and other applications.
KEYWORDS:Zinc suplhidenanoparticles; Tridaxprocumbens; Antimicrobial Activity; Scanning electron microscopy (SEM) and X-ray powder diffraction (XRD)
Download this article as:| Copy the following to cite this article: Sathishkumar M, Saroja M, Venkatachalam M, Parthasarathy G, Rajamanickam A. T. Biosynthesis and Characterization of Zinc Sulphide Nanoparticles using Leaf Extracts of Tridaxprocumbens. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Sathishkumar M, Saroja M, Venkatachalam M, Parthasarathy G, Rajamanickam A. T. Biosynthesis and Characterization of Zinc Sulphide Nanoparticles using Leaf Extracts of Tridaxprocumbens. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31683 |
Introduction
From last decades, nanoparticles have been received too much attention due to its unique properties like high surface area, magnetic, thermal, mechanical, electronic and enhanced catalytic properties. The nanotechnology has been remarkable development in the synthetic methodologies have been developed to various nanoparticles of shape, size, dimensions and so on (1-2). For instance, various types of nanoparticles (NPs) are available such as metal NPs (3), carbon based nanomaterials (4-7), and polymers (8), most commonly nanoparticles are synthesis through chemical methods (9), sol-gel processing (10), co-precipitation, and electro deposition method (11). Zinc sulphide has been found highly attractive because of its remarkable application potential in solar cells, piezoelectric devices, UV-vis absorbers, pharmaceutical and cosmetic industries (12). Potentially, Zinc sulphide removed all the dyes and water pollutants from textile effluent under UV light have been proved (13-15). Zinc sulphide nanoparticles were large particle size in bulk materials, morphology and characteristics of the particles can be derived due to their new properties (16). The amount of the atoms and size approaches nanoscale materials become memorable of their properties (17). In pharmaceutical applicationsbiosynthesis of zinc sulphide nanoparticles using green chemistry, amethod is very useful and eco friendly environmental method (18). Although different biological based synthetic method is known for zinc sulphide are sought by researchers. Biosynthesis of zinc sulphide nanoparticles has many advantages in pharmaceuticals applications because of use nontoxic materials (19). Plants become the desirable and superior option for the safe, Nontoxic method of nanoparticles synthesis because the natural capping agents are readily taken from the plants (20).Tridaxprocumbens is belonging to family Asteraceae. It’s grown on waste lands and hill stations normally, the leaves and flowers are commonly toothed and arrowhead shaped, yellow colored flowers, Tridaxprocumbens plant has more herbal properties in pharmaceuticals(21-22).Coat buttons is a common name of Tridaxprocumbens in English because of its flower appearance. In India its widely used ayurvedic medicine extensively like wound healing, liver disorders. Especially leaves are used skin infection (23). Antiseptic, Parasiticidal, Insecticidal infections flowers and leaves also used (24-26). Tridaxprocumbens full plants show various antibacterial and fungal activities against many microorganisms and various pharmacological activities (27-32).
Materials and Methods
Plant Extraction
Tridaxprocumbens plant fresh leaves were collected locally around the waste land of idappadi, Salem. Collected leafs washed in DI water and paced shadow dried, coarsely powdered using agrinder. 250 ml of methanol solvent, 25 g of coarse leaves powder to extracts by using soxhlet apparatus and stored extracts used further investigations.
Alkaloids Detection
The hydrochloric acid used to test a presence of alkaloids, Tridaxprocumbens leaf extracts were dissolved in HCL and the extract was filtered. The presence of alkaloids to expedite yellow cream formation were filtrated extracts with Mayer’s reagent and the presence of alkaloids to precipitate brown or reddish brown colour formation were filtrates with Wagner’s reagent.
Flavonoids Detection
The presence of flavonoids to expedites yellow colour formation were a solution of lead acetate treat with drops of extracts and H2SO4 were treating with leaf extract, orange colour formation to the presence of flavonoids.
Steroids Detection
Presences of steroids were leaf extract added 1ml of acetic anhydride with H2SO4 expedites blue or green from violet colour.
Terpenoids Detection
In salkowski’s test presence of terpenoids, indicate reddish brown were leaf extract was mixed chloroform with H2SO4
Athraquinones Detection
In borntrager’s test presence of anthraquinones expeditespink colour, were NH3 drops was adding cool
Filtered with CHCl3 and before filtering extract was boiled HCL using awater bath in few minutes.
Phenols Detection
In Ferric chlorideand lead acetate test presence of phenol indicates bluish black colour formation were leaf extracts mixing with ferric chloride drop by drops,yellow colour expedites were solution of lead acetate was adding in the extract
Saponins And Tannins Detection
The presence of saponins was extract shaken with DI water itindicate a frothing formation when ferric chloride added to filtrate of mixing heated water bath with extract to the formation of dark green colour indicate of tannins.
Carbohydrates, oil and Resins Detection
DI water was dissolved in extract and filtered carbohydrate presence was tested using filtrate. Were the test solution was applied on the filter paper its form a transparent appearance to indicate the presence of oil and resins.
Alkaloids Estimation
Harborne(1973) method to use determined of alkaloid, 200 ml acetic acid was taken in 250ml of the beaker and 4 to 5g extract were added together and the beaker allowed to stand more then 4h after that extract was filtered and treated to a water bath with original volume. Ammonium hydroxide was added drop by drop of the extract until it was complete. Once extracts were washed with dilute ammonium hydroxide solution it’s filtered and weighed.
Flavonoids Estimation
The filter paper was use to dry and weighed of flavonoids, In 250 ml beaker uses to mixing 100ml of methanol and 10g of leaf extract at room temperature.
Steroids Estimation
2 ml of sulphuric acid, 2 ml of iron chloride was added and 5ml of potassium hexacynoferrate solution were mixed with 10 ml of tridaxprocumbens leaf extract, followed mixing were treated heating water bath at 200 C to 700 C in 30 minutes , then the mixed solution dissolved in DI water.
Saponin Determination
The saponin content calculate using the methanol, 10 ml methanol were treated with 10 ml tridaxprocumbens plant extract it was dispersed and solution was using continuous stirring 4 hours at 550C With heated hot water, after complete stirring solution was filtered and again 200 ml methanol added. In that solution was heated till it reduce 90 ml using heat water bath, from the 90 ml solution 20 ml of diethyl added and shacked with separate funnel and purification process was taken. In that purified solution was adding with 10 ml of sodium chloride and remaining was treated water bathing and dried with help of oven.
Apparatus and Synthesis
The surface morphology and EDX of the composite were studied by Hitachi S-4500 scanning electron microscope (SEM). Fourier transform infrared spectroscopy (FT-IR) was measurements were carried out by using JASCO FT/1 IR-6600 instrument. XRD pattern were analyzed by X-ray diffractometer (XPERT-PRO).
Biosynthesis of Zinc Sulphide Nanoparticles
Tridaxprocumbens used to synthesis zinc sulphide nanoparticles were 14.4 g of zinc sulphate was added 100 ml DI water, one millimole aqueous solution of zinc sulphate (ZnSO4) was prepared, 20 ml of methanol leaf extract were added drop by drop in 80 ml(1mM) of zinc sulphate aqueous solution which was kept continuous stirring for 2 hours at room temperature, result was indicate the formation of zinc sulphide nanocolloid, then the final solutiondried at 600 C for 5 hours.
Result and Discussion
XRD Pattern
The crystalline structural properties of prepared biosynthesis of ZnS nanoparticles powder sample is studied using X-ray diffraction figure 1 shows the different peak pattern of synthesized ZnS at 2θ value of 31.820,34.630,36.070,48.720,54.040 and 63.050 and corresponding reflection planes is 100,002,101,102,110 and 103 respectively it indicate Hexagonal Wurtzite structure.
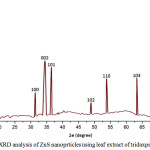 |
Figure 1:XRD analysis of ZnS nanoprticles using leaf extract of tridaxprocumbens Click here to View figure |
Debye Scherrer’s equations were used to calculate particle crystalline grain size of the ZnS.
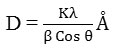
Here D is the mean of grain size, K= 0.9 constant value, is x-ray wavelength for CuKa radiation, Ɵ is the diffraction angle in radian, β is full with half maximum (FWHM) in radian so the average grain size of synthesis zinc sulphide is ~ 40 nm
Morphology Studies
Figure 2 clearly shows the surface morphology of biosynthesized zinc sulphide nanoparticle powder were examined and found different radial hexagonal, spherical, rod shapes and some surface areas various size of hexagonal shape particles observed and their size was found to be less than 5µm.
 |
Figure 2: SEM analyses of ZnS nanoparticles using leaf extract Tridaxprocumbens Click here to View figure |
UV- Visible Analysis
The optical absorption of biosynthesized zinc sulphide nanoparticle clearly shown in figure 3 and absorption range 200 – 400 nm, the maximum absorption occurred at 270 nm.
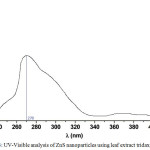 |
Figure 3: UV-Visible analysis of ZnS nanoparticles using leaf extract tridaxprocubens Click here to View figure |
FT-IR Spectrum
The FTIR analysis of zinc sulpide nanoparticles synthesized using tridaxprcumbens methanol leaf extract showed in figure 4, ranging from 500 – 4000 cm-1 wave numbers and its produces zero to two N-H absorptions depending on its type at 3850.50 cm-1, Alkenyl C-H stretch at 3101.94 cm-1, Alkenyl C=C stretch at 1642.05 cm-1, Aromatic C=C Bending at 1502.47 cm-1, Polysaccharides at 1091.33 cm-1 and C-H Vibration at 594.62 cm-1 according to this stretching ZnS nanoparticles have degradation of TGA
 |
Figure 4: FTIR analysis of ZnS nanoparticles using leaf extract Tridaxprocumbens |
Antimicrobial Activity of ZnS
Muller Hinton Agar (MHA) plate from Hi-media Mumbai very useful to screening antimicrobial activity, Here disc diffusion method was helpful to analysis antimicrobial activity. Before swap inoculums in the MHA plate surface it was treated 15 ml molten media in to sterile petriplates from pouring process and allowed 5 minutes for solidify then the minimum amount of (0.1%) microorganisms suspension was transferred uniformly in MHA plate surface and allowed 5 minute for drying , then 60 mg concentration disc placed on 6 mm sterile disc that was palced in surface of MHA plates for allowing 5 minutes to diffuse the ZnS leaf extract solution, after diffused that MHA plates are placed incubation at 370C for 24 hours , during the incubation zone of inhibition formed around the sterile disc and zone was calculate by using transparent millimeter ruler. The phytochemical analysis of methanol leaf extract of tridaxprocumbens zinc sulphide was clearly indicate the Table 1 and the plant found alkaloids,flavonoids,phenol,crbohydrates,steroids were absent in Terpenoid,anthrouinone,saponins,tannins oil and resins. In this different functional which was helpful to react with zinc sulphide and increase a zone of inhibitions, FTIR analysis shows different function group bonding to enter the cell membrane of inoculums. The quantitative analysis by presence and absence of inhibition zone, methanol leaf extract of zinc sulphide using tridaxprocumbns has excellent antimicrobial activity against all tested microorganisms its clearly shown in figure 5. It was tested both gram positive, gram negative bacteria’s and fungal culture by disc diffusion method the zone of inhibition is formed antibiotic action of zinc sulphide nanoparticles. Table 2 clearly indicate antimicrobial activity of all tested microorganism with different concentration at 30 µl, 40 µl, 50 µl and 60 µl, in this six microorganisms Staphylococcus aureus, Bacillus subtilis are gram positive and Escherichia coli, Pseudomonas aeruginosa are gram negative and Candida albicans, Aspergillus niger is fungus culture, at a concentration 30 µl P.aeruginosa start a high zone of inhibition (15 mm) followed by S.aureus and E. coli (14 mm), minimum zone of inhibition was found in B.subtilis (8 mm) from bacterial culture but in a fungus culture C.albicans (11 mm) and A.niger (10 mm) here C.albicans lead high zone of inhibition to compare A.niger.
Table 1: Qualitative phytochemical analysis of ZnS nanoparticles using leaf extract of Tridaxprocumbens
| Phytochemicals | Observations | Extracts |
| Methanol | ||
| AlkaloidsMayer’s testWagner’s test | Cream colorReddish brown solution/ precipitate | ++ |
| FlavonoidsLead acetate testH2SO4 test | Yellow orangeReddish brown / Orange color precipitate | ++ |
| Steroids Liebermann-Burchard test | Violet to blue or Green color formation | + |
| TerpenoidsSalkowski test | Reddish brown precipitate | – |
| ArthroquinoneBorntrager’s test | Pink color | – |
| PhenolsFerric chloride testLead acetate test | Deep blue to Black color formationWhite precipitate | ++ |
| Saponin | Stable persistent | – |
| Tanin | Brownish green / Blue black | – |
| Carbohydrates | Yellow / brownish / blue / green color | + |
| Oils & Resins | Filter paper method | – |
‘+’ Present, ‘-‘Absent
The concentration 40 µl and 50 µl same zone of inhibition was increased in both bacterial and fungal culture but concentration 50 µl B.subtils inhibition zone was increased as the same of A.niger fungus culture, S,aureus inhibition zone also increased to compare E.coli, but the maximum zone was found in P.aeruginosa at 40 µl and 50 µl concentration.
Table 2: Antimicrobial activity of ZnS nanoparticles using leaf extract of Tridaxprocumbens
|
Organism |
Different concentration of ZnS samples |
||||
|
C |
30 µl |
40 µl |
50 µl |
60 µl |
|
| S.aureus |
26 |
14 |
15 |
18 |
20 |
| B.subtilis |
25 |
8 |
10 |
13 |
18 |
| E.coli |
24 |
14 |
16 |
17 |
20 |
| P.aerugoinosa |
28 |
15 |
16 |
19 |
22 |
| C.albicans |
24 |
11 |
14 |
18 |
20 |
| A.niger |
25 |
10 |
11 |
13 |
15 |
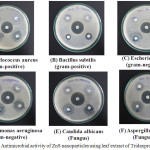 |
Figure 5: Antimicrobial activity of ZnS nanoparticles using leaf extract of Tridaxprocumbens Click here to View figure |
Finally the maximum zone of inhibition were observed at 60 µl concentration for P.aeruginosa (22 mm) and minimum zone of inhibition was observed B.subtilis (18 mm) and A.niger (15 mm), so the in vitro antimicrobial activity of biosynthesized zinc sulphide leaf extract was high excellent activity against all tested microcrganisms, the tridaxprocumbens various plant parts are used to found antimicrobial agents(33)there are so many plants are have high antimicrobial activity because of their zone of inhibition against bacteria and fungus (34) Biosynthesis is good and new field of research because it’s from natural sources(35).
Conclusion
In conclusion, we have reported a biosynthesized of zinc suphide nanoparticles using methanol leaf extract tridaxprocumbens for the first time, and the structural characterization and morphology of the zinc sulphide nanoparticles analyzed using XRD and SEM, the ZnS nanoparticles are Hexagonal wurtzite structure and average grain size of the nanoparticles is 40 nm and surface morphology was found various size of nanoparticles blow 5µm, the optical observation of the colloidal solution were studied using UV-Visible the maximum absorption frequency was 270 nm and FTIR very useful to found different functional group present in the leaf extract solution. Further antimicrobial activity of tridaxprocumbens methanol leaf extract and synthesized zinc sulphide nanoparticles were investigated in the disc diffusion method from the result it’s clear to known that the zinc sulphide nanoparticles have excellent zone of inhibition in the all tested pathogenic microcoranisms like Staphylococcus aureus , Bacillus subtilis are gram positive and Escherichia coli, Pseudomonas aeruginosa are gram negative and Candida albicans, Aspergillus niger is fungus culture. The maximum zone of inhibition was observed in Pseudomonas aeruginosa (22 mm) in 60 µl concentration and minimum was observed in Aspergillus niger(15 mm), it was evident that the methanol leaf extract tridaxprocumbens can be used to synthesis ZnS nanoparticles using green chemistry method for various applications.
Reference
- Manojkumar, K.; Sivaramakrishna, A.; Vijayakrishna, K. J Nanopart Re. 2016, 18,103
- Chang, CC.; Chiang, FC.; Chen, SM.; Thangavelu, K; Yang, HJ Int. J. Electrochem. Sci. 2015, 11 (3), 2142-2152
- Palanisamy, S.; Thangavelu, K.; Chen, S.M. ; Thirumalraj, B.; Liu, X.H. Sens. Actuators, B. 2016, 233, 298–306.
CrossRef - Palanisamy, S. ; Thangavelu, K.; Chen, S.M.; Velusamy, V.; Chen, TE.; Kannan, RS J. Electroanal. Chem. 2017,785, 40–47.
CrossRef - Thangavelu, K.; Palanisamy, S.; Chen, S.M.; Velusamy, V.; Chen, T.W. ; Kannan, R.S. J. Electrochem. Soc. 2016 ,163(14,726-731.
- Palanisamy, S.; Thangavelu, K.; Chen, S.M.; Gnanaprakasam, P.; Velusamy, V.; Liu, X.H. carbohydr. Polym.2016, 151, 401-407.
CrossRef - Thirumalraj, B.; Palanisamy, S.; Chen, S.M.; Thangavelu, K.; Periakaruppan, P.; Liu, X.H. J. Colloid Interface Sci. 2016, 475, 154–160.
CrossRef - Palanisamy, S.; Thangavelu, K.; Chen, S.M.; Velusamy, V.; Chang, M.H.; Chen, T.W.; AlHemaid, F.M.A.; Ali, M.A.; Kannan, R.S. Sens. Actuators, B. 2017, 243, 888–894.
CrossRef - Ameer, A.; Faheem, A.; Nishat, A.; Chaman, M.; Naqvi, A.H. Jou. Alloy. Com.2010, 496, 399-402.
- Alessio, B.; Maximilian, D.; Pierandrea, L., Nostro. PB. Journal of Nanopart Res.2007, 10, 679-689.
- Surabhi, S.; Putcha, V.; Vankarango, R.; and Gollapallinageswsara. R. International nano letters. 2013, 3, 30.
- Grasseta, F.; Saitoa. N.; Lia, D.; Parka, D.; Sakaguchia, I.; Ohashia, N.; Hanedaa, H.; Roisnelc, T.; Mornet, S.; Duguet, E. Jou. Alloys. Com. 2003, 360 , 298-311.
- Deepti, K.; Pradeep, T. Jou. Crystal growth.2009, 311-3889.
- Li, M.; Bala, H.; Lv, X. Jou. Mate. Lett.2007, 61-690.
- Xiaoxia, Lin.; FeiRong.; Degang, Fu.; Chunwei, Yuan. Jou. Powder Tech.2012, 219, 173-178.
- Pieqiang,Li.; Guohua, Zhao. Jou. Dye and pigments.2012,92, 923-928.
- Ravindra, P.; Singh, V.K.; Shukla.; Raghvendra, S.; Yadav, P.K.; Sharma, P.K.; Avinash, C.P. Jou. Advanced Materials Letters. 2011,. 2,.4, 313-317.
- Harish, K. H.; PrashanthKumar, J.; Shruthi, SD. Spreng. Pharmacophore.2010, 13, 231-238.
- Akl, M.; Awwad, N.M.; Amany. O. Jou.Nanoscience and Nanotechnology.2012, . 2012, 2(6), 164-170.
- Sigh. R. P.; Magesh. S.; Rakkiyappan. C. Jou. Nanotechnology and application. 2012, 6, 43- 51.
- Ingale. G.; Chaudhari, A. N. Jou. Nanomedicine and Nanotechnology. 2013, 4, 1-7.
- Sahoo. M.; Chand. P. K. Jou. Phytomorphology. 1998, 48, 195 – 206.
- Ali. M.; Ravinder. E.; Ramachandram. R. Jou. Fitoterapia.2001, 72, 313-315.
- Ravikumar. V.; Shivashangari. K.S.; Devaki.T. Jou. Mol. Cell Biochem. 2005, 269, (1-6).
- Vyas . P. S.; Tiwari . U.; Rastogi. B.; Singh. P.; Journal of Ethnopharmacologoly. 2004, 92, 113-119.
- Bhagwat. D.A.; Killedar, Suresh. G.; Adnaik. R.S. International Journal of Green Pharmacy. 2010, 2(2), 126-128.
- Deepali. S; Sapna. S.; Kaitha. B. S.; Jaspreet. R.; Mohinder. K.; Applied Surface Science. 2011, 257, 9661-72.
- Bibitha. B.; Jisha. V.K.; Salitha. C.V.; Mohan. S. Journal of Current Agriculture. 2002, 28, 75-78.
- Maghrani. M.; Zeggwah. N.; Michel. J.; Eddouks. M. Jou. Ethnopharm.2005, 102(1-2), 193-197.
- Ravindra. S. Physicochemical and Engineering Aspects2010, 367.1, 31-40.
- Padmavati. N.V.; Vijayaraghavan. R. Sci.Technol.Adv.Mater. 2008, 9, 1468.
- Meena. W.; Shubha. Jain. Jou.Mat,Sci.2015, 04, 36-39.
- Vanaja. M.; Rajeshkumar. S.; Paulkumar. K.; Gnanajobitha. G.; Malarkodi. C.; Annadurai. G. International Journal of Materialsand Biomaterials Application. 2013, 3(1), 1–4.
- Malarkodi. C.; Annadurai. G. Jou. Applied Nanoscience. 2013,3(5), 389–395.
- Warad. H. C.; Ghosh. S. C.; Hemtanon. B.; Thanachayanont. C .D. Jou. Sci.Technol.Adv. Mater. 2005, 6, 296.
- Baishya. U.; Sarkar. D. Indian Jou. ofpure& Applied Physics. 2011, 49, 186-189.
- Thakkar. K.N.; Mhatre. S.S.; Parikh. R.Y. Jou.Nanomed.2010, 6, 257-262.
- Sheng. Xu.; Zhong. L.W. Jou.Nanoresearch. 2011,4(11), 1013-1098.

This work is licensed under a Creative Commons Attribution 4.0 International License.









