Bicl3: A Versatile Catalyst for the Tetrahydropyranylation and Depyranylation of 1°,2°,3°,Allylic,Benzylic Alcohols, and Symmetric Diols
T. Vijaya Durga1, K. Bala Murali Krishna1, M. Baby ramana1, M. Shantha Kumari2, K. Vijay3 and B. Hari Babu1
1Department of Chemistry, Acharya Nagarjuna University, NNagar-522 510, Aandra Predesh,-India.
2Department of Chemistry, CRR Degree and PG College, Eluru, W.G. Dist, Aandra Predesh - India.
3College of Pharmaceutical sciences, Acharya Nagarjuna University, NNagar-522 510, Aandra Predesh - India.
Corresponding Author E-mail: dr.b.haribabu@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330260
Bismuth trichloride as mild reagent, has been found to be a worthful catalyst for tetrahydropyranylation of 1º, 2 º, 3º, allylic, benzylic alcohols, and symmetric di-ols. At room temperature the reagent THP(3,4–dihydro-2H-pyran) was successfully employed as pyranylating agent in presence of BiCl3 catalyst without the use of a solvent and the yields of the products were found to be 90-96%. Further, the depyranylation of alcohols was achieved in quantitative yield by simple addition of MeOH using the same catalyst. The developed method was showed good chemo-selectivity in symmetrical diols for mono THP protection.
KEYWORDS:Bismuth trichloride; tetrahydropyranylation; depyranylation; alcohols; symmetrical diols
Download this article as:| Copy the following to cite this article: Durga T. V, Krishna K. B. M, ramana M. B, Kumari M. S, Vijay K, Babu B. H. Bicl3: A Versatile Catalyst for the Tetrahydropyranylation and Depyranylation of 1°,2°,3°,Allylic,Benzylic Alcohols, and Symmetric Diols. Orient J Chem 2017;33(2). |
| Copy the following to cite this URL: Durga T. V, Krishna K. B. M, ramana M. B, Kumari M. S, Vijay K, Babu B. H. Bicl3: A Versatile Catalyst for the Tetrahydropyranylation and Depyranylation of 1°,2°,3°,Allylic,Benzylic Alcohols, and Symmetric Diols. Orient J Chem 2017;33(2). Available from: http://www.orientjchem.org/?p=31609 |
Introduction
While doing multi stage organic synthesis, the protection and deprotection of alcohols by various reagents is a most common event. The most common protected forms of alcohols are ethers and esters, where as in case of alkyl and benzyl ethers the protection is permanent (difficult to remove); and remaining such as TBS, TPS, THP, are acid labile (easier to remove). Among the reagents the protection of alcohols and phenols by THP reagent is one of the important and frequently used approach1 as the preparation of the reagent is easy and stable in various reaction conditions such as with alkylating reagents, hydrides, Grignard reagents and organo metallic agents2.
A large number of reagents such as BF3.Et2O,3 PPTS,4 Amberlyst,5 PTSA,6 Nafion-H,7 montmorillonite K-10,8 ZnCl2,9 AlPO4 on alumina,10 lithium perchlorate-diethyl ether,11 zeolites,12 sulfuric acid on silica-gel,13 Bronsted acids14 ZrCl4,15 iodine,16 LiBr,17 tetrabutylammonium bromide,18 Lewis acids,19 In(OTf)3,20 and NbCl521 are useful for the tetrahydro-pyranylation of alcohols. Recently an organic reagent 3,5-dinitrobenzoic acid,was also employed for tetrahydropyranylation and depyranylation for alcohols and phenols22. However, many of the above reported procedures suffers from use of toxic and expensive reagents, need of large amount of catalyst, presence of acid-sensitive functional groups, low chemo-selectivity, and unsatisfactory yields. Moreover, the reaction conditions such as higher temperatures, longer reaction times, strong oxidizing and acidic conditions, also futile the methods. Hence there is a strong requirement for novel catalytic approach to the protection and deprotection of hydroxyl functional groups in the absence of solvent. Bismuth trichloride (BiCl3) has emerged as a catalyst in organic synthesis23 and attracted attention of many researchers because of its diverse applicability. Moreover, bismuth(III) salts are stable in air, relatively inexpensive and nontoxic compared to transition metal complexes. So, in the present study BiCl3 is employed as a catalyst for pyranylation and depyranylation of alcohols and symmetric diols (Scheme 1).
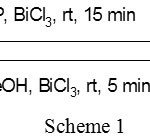 |
Scheme 1 Click here to View scheme |
Experimental
Materials and Methods
All the chemicals were obtained from Merck Specialties Private Limited, Mumbai, India. Reactions were monitored by TLC using silica gel-G (Merck grade) as the adsorbent. Silica gel (100-200 mesh, Merck grade) has been used for column chromatography.
The 1H NMR spectra of the compounds dissolved in duterated chloroform (CDCl3) were recorded on Bruker AMX 300 MHz NMR spectrophotometer using TMS as an internal standard and the values are expressed in δ ppm. The Mass spectra of the compounds were recorded on API-ES Mass Spectrometer using positive mode ionization method.
General Procedure for Tetrahydropyranylation
To a mixture of 1º, 2º, 3º, benzylic, allylic alcohol or symmetric diol (1 mmol) and THP (1 mmol), bismuth trichloride (5 mol %) was added.then the reaction mixture was stirred for appropriate time as given in Table 1 at room temperature. The reaction as monitored by TLC. After completion of the reaction it was poured into water and the product was extracted with ethyl acetate (3×25 mL). Later the organic layer was washed with brine, dried with anhydrous sodium sulphate and concentrated in vacuum to obtain the crude mass. Which was further purified by silica gel column chromatography(1:9 EtOAc, hexane) to afford the corresponding THP ether. Finally the products were characterized using 1H NMR and Mass spectra.
General Procedure for Depyranylation
To the above THP ether solution(1 mmol) in methanol (10 mL), 5 mol % bismuth trichloride was added. The reaction mixture was stirred for appropriate time as provided in Table 1 at room temperature. the reaction as monitored by TLC. After completion of the reaction water was added to the reaction mixture and the product was extracted with ethyl acetate (3×25 mL). Later the combined organic layer was washed with brine, dried over anhydrous sodium sulphate and concentrated in vacuum to give a crude mass, which was purified over silica gel column to afford parent alcohol in quantitative yield.
Analytical Data
Compound 1
1H NMR, δ = 1.43-1.90 (m, 6H), 2.90 (b s, 1H, OH), 3.52 (m, 1H), 3.85 (m, 1H), 4.02-4.24 (m, 4H), 4.68 (t, 1H), 5.58-5.84 (m, 2H); EIMS: m/z 172 (M+).
Compound 2
1H NMR, δ = 1.50-1.90 (m, 6H), 3.47 (m, 1H), 3.82 (m, 1H), 3.89-3.97 (m, 1H), 4.15-4.23 (m, 1H), 4.60 (t, 1H), 5.12-5.16 (m, 1H), 5.23-5.30 (m, 1H); EIMS: m/z 142 (M+).
Compound 3
1H NMR, δ = 1.50-1.90 (m, 6H), 3.45-3.53 (m, 1H), 3.60-3.70 (m, 3H), 3.80-3.90 (m, 2H), 4.65 (t, 1H); EIMS: m/z 154 (M+).
Compound 4
1H NMR, δ = 0.90 (t, 3H), 1.40 (m, 2H), 1.50-1.90 (m, 6H), 2.00 (m, 2H), 3.50 (m, 1H), 3.70 (m, 2H), 4.10 (m, 1H), 4.62 (t, 1H), 5.02-5.21 (m, 2H); EIMS: m/z 184 (M+).
Compound 5
1H NMR, δ = 1.13 (d, 3H, J = 6.7 Hz), 1.20 (d, 3H, J = 6.0 Hz), 1.40-190 (m, 6H), 3.45 (m, 1H), 3.90 (m, 2H), 4.62 (t, 1H); EIMS: m/z 144 (M+).
Compound 6
1H NMR, δ = 1.18-1.94 (m, 16H), 3.50 (m, 2H), 3.82 (m, 1H), 4.64 (t, 1H); EIMS: m/z 184 (M+).
Compound 7: 1H NMR, δ = 1.48-1.88 (m, 6H), 3.50 (m, 1H), 3.85 (m, 1H), 4.10 (m, 1H), 4.35 (m, 1H), 4.67 (t, 1H), 6.25 (m, 1H), 6.60 (d, 1H, J = 15.8 Hz), 7.45 (m, 5H); EIMS: m/z 216 (M+).
Compound 8
1H NMR, δ = 1.40-1.90 (m, 6H), 2.85 (t, 2H), 3.40 (m, 1H), 3.62 (m, 2H), 3.80 (m, 1H), 4.52 (t, 1H), 7.20 (m, 5H); EIMS: m/z 206 (M+).
Compound 9
1H NMR, δ = 1.47-1.58 (m, 4H), 1.60 (s, 3H), 1.68 (s, 6H), 1.82 (m, 2H), 2.10 (m, 4H), 3.50 (m, 1H), 3.82 (m, 1H), 4.00 (m, 1H), 4.20 (m, 1H), 4.60 (t, 1H), 5.06 (t, 1H), 5.30 (t, 1H); EIMS: m/z 238 (M+).
Compound 10
1H NMR, δ = 1.40-1.90 (m, 6H), 2.45 (m, 2H), 3.41 (m, 1H), 3.85 (m, 1H), 4.35 (t, 1H), 4.64 (t, 1H), 5.00 (m, 2H), 5.75 (m, 1H), 7.20 (m, 5H); EIMS: m/z 232 (M+).
Compound 11
1H NMR, δ = 1.5 (s, 9H), δ = 1.7 (m, 2H), δ = 3.3(m, 2H), δ = 3.8 (m, 2H), δ = 4.5 (m, 2H), δ = 4.9 (t, 1H); EIMS: m/z 158.13 (M+).
Results and Discussion
The present investigation describes a mild and efficient method for the tetrahydropyranylation of 1º, 2 º, 3º, benzylic, allylic alcohol and symmetric diols with THP in the presence of bismuth trichloride (5 mol %) as catalyst without the use of a solvent at room temperature. In intial trails THP (3,4-dihydro-2H-pyran) was reacted with cinnamyl alcohol (scheme 1) as parent alcohol using 5 mol% of bismuth trichloride at room temperature to produce its corresponding THP ether of cinnamyl alcohol. A very good yield (96%) of the product was found and the same trend was continued in the remaining tested alcohols (Table 1).
 |
Table 1: Tetrahydropyranylation and depyranylation of alcohols
|
The depyranylation of the above obtained THP ether was easily obtained by maintaining the same quantities of the reagent in methanol at room temperature. All the deprotections were found to be completed within 5 min. The results of all the compounds were also illustrated in Table 1. The yields of depyranylation products are also good(92-98%).
We claim that the developed protocol is quite general as it is applicable to a large number of structurally different alcohols viz. primary, secondary, tertiary, allylic and benzylic alcohols. The method was also found to be highly selective for mono tetrahydro-pyranylation of symmetrical diols. The compound 11(Table 1) an acid sensitive alcohol was underwent THP protection as tetrahydropyranyl ether with 95% yield and no dehydration product was observed. So, from these results (Table 1) it is evident from these results that bismuth trichloride is an excellent catalyst for tetrahydropyranylation and depyranylation of alcohols under solvent-free conditions.
Conclusions
The present protocol developed in this work demonstrates that bismuth trichloride is an efficient catalyst for the tetrahydropyranylation and depyranylation of alcohols. The major advantages of this protocol is use of mild catalyst, clean and environmentally benign reaction conditions. The less reaction times and good yields of the products are additional advantages. Simple experimental work-up procedure and mono tetrahydro-pyranylation selectively of symmetric diols offer significant improvements, low cost of the reagent.
Acknowledgements
We thank Acharya Nagarjuna University for encouragement and UGC, New Delhi [F.No. 25-1/2014-15(BSR)/11-67/2008(BSR) dated 25-08-2015] for providing Fellowship to the first author. We also thankful to CSIR, New Delhi for financial support to some chemicals (EMR-II/02(198) dt. 17.11.2014).
References
- Greene, T. W.; Wuts, P. G. M. Protective Groups in Organic Synthesis, 3rd ed.; John Wiley and Sons: NewYork, 1999; 49-54.
- Sartori, G.; Ballini, R.; Bigi, F.; Bosica, G.; Maggi, R.; Righi, P. Chem. Rev. 2004, 104, 199-250.
CrossRef - Alper, H.; Dinker, L. Synthesis 1972, 81-82.
- Miyashita, M.; Yoshikoshi, A.; Grieco, P. A. J. Org .Chem. 1977, 42, 3772-3774.
- Bongini, A.; Cardillo, G.; Orena, M.; Sandri, S. Synthesis 1979, 618-620.
- (a) Corey, E. J.; Niwa, H.; Knolle, J. J. Am. Chem. Soc.1978, 100, 1942-1943; (b) Bernardy, K. F.; Floyd, M. B.; Poletto, J. F.; Weiss, M. J. J. Org. Chem. 1979, 44, 1438-1447.
- Olah, G. A.; Husain, A.; Sing, B. P. Synthesis 1983, 892-895.
- Hoyer, S.; Laszlo, P.; Orlovic, M.; Polla, E. Synthesis 1986, 655-657.
- Ranu, B. C.; Saha, M. J. Org. Chem. 1994, 59, 8269-8270.
CrossRef - Campelo, J. M.; Garcia, A.; Lafont, F.; Luna, D.; Marinas, J. M. Synth. Commun. 1994, 24, 1345-1350.
CrossRef - Babu, B. S.; Balasubramanian, K. K. Tetrahedron Lett. 1998, 39, 9287-9288.
CrossRef - (a) Rodriguez, I.; Clement, M. J.; Iborra, S.; Fornes, V.; Corma, A. J. Catal. 2000, 192, 441-447; (b) Kumar, P.; Dinesh, C. U.; Reddy, R. S.; Pandey, B. Synthesis 1993, 1069-1070; (c) Ballini, R.; Bigi, F.; Carloni, S.; Maggi, R.; Sartori, G. Tetrahedron Lett. 1997, 38, 4169-4172.
- Heravi, M. M.; Ajami, D.; Ghassemzadeh, M. Synth. Commun. 1999, 29, 1013-1016.
CrossRef - (a) Nishiguchi, T.; Fujisaki, S.; Kurodo, M.; Kajisaki, K.; Saitoh, M. J. Org. Chem. 1998, 63, 8183-8187; (b) Branco, L. C.; Afonso, C. A. M. Tetrahedron 2001, 57, 4405-4410; (c) Ravindranath, N.; Ramesh, C.; Das, B. Synlett 2001, 1777-1778; (d) Romanelli, G. P.; Baronetti, G.; Thomas,H. J.; Autino, J. C. Tetrahedron Lett. 2002, 43, 7589-7591.
- Rezai, N.; Meybodi, F. A.; Salehi, P. Synth. Commun.2000, 30, 1799-1805.
CrossRef - Deka, N.; Sarma, J. C. Synth. Commun. 2000, 30, 4435-4441.
- Reddy, M. A.; Reddy, L. R.; Bhanumathi, N.; Rao, K. R. Synth. Commun. 2000, 30, 4323-4328.
CrossRef - Naik, S.; Gopinath, R.; Patel, B. K. Tetrahedron Lett. 2001, 42, 7679-7681.
CrossRef - (a) Nambodiri, V. V.; Varma, R. S. Tetrahedron Lett. 2002, 43, 1143–1146; (b) Karimi, B.; Maleki, J. Tetrahedron Lett. 2002, 43, 5353-5355.
- Mineno, T. Tetrahedron Lett. 2002, 43, 7975-7978.
- (a) Kavala, V.; Samal, A. K.; Patel, B. K.; Nagaiah, K. ARKIVOC, 2005, 1, 22-29; (b) Nagaiah, K.; Reddy, B. V. S.; Sreenu, D.; Narsaiah, A. V. ARKIVOC, 2005, 3, 192-199.
- Sivarama Krishna Reddy, v.; Satyannarayana, P.V.V.; IOSR JAC 2014, 7, (38-43).
- For comprehensive reviews: (a) Postel, M.; Dunach, E. Coord. Chem. Rev. 1996, 155, 127–144; (b) Leonard, N. M.; Wieland, L. C.; Mohan, R. S. Tetrahedron 2002, 58, 8373–8397; (c) Vidal, S. Synlett 2001, 1194–1195; (d) Hua, R. M. Curr. Org. Synth. 2008, 5, 1–27.

This work is licensed under a Creative Commons Attribution 4.0 International License.









