Vermiculite Absorption Capacity Increasingby Acid Activation
Kulash K. Syrmanova1,2, Moldir T. Suleimenova2, Anastassiya Y. Kovaleva1, Nurzhan Y. Botabayev1 and Zhanat B. Kaldybekova1
1Auezov South Kazakhstan State University, Kazakhstan, 160012, Shymkent, Tauke Khan av., 5.
2“MIRAS” University, Kazakhstan, 160012, Shymkent, Ilyaevst., 5.
Corresponding Author E-mail: anastasiya2301@mail.ru
DOI : http://dx.doi.org/10.13005/ojc/330160
The article is devoted to the research of the acid activation process of mineral sorbent – vermiculite. This layered silicatescould be applied for adsorptive separation and purification of chemical substances in petrochemical, gas, food, pharmaceutical and chemical industry. On the basis of X-ray diffraction and adsorption it was shown that the adsorption properties, and other physical and chemical properties of the layered silicates with a rigid structure are defined by dispersion, which in turn depends on the vermiculitecrystal lattice structure. It was proved that the formation of amorphous silica during the vermiculite treatment with hot sulfuric acid. Vermiculite can be used to produce technical silica, because the activation by solution of 20-25% H2SO4occurs total destruction of its lattice and the formation of substantially pure silica.
KEYWORDS:adsorbent; vermiculite; acid activation; mineral sorbent; crystal lattice; porosity; sulfuric acid
Download this article as:| Copy the following to cite this article: Syrmanova K. K, Suleimenova M. T, Kovaleva A. Y, Botabayev N. Y, Kaldybekova Z. B. Vermiculite Absorption Capacity Increasingby Acid Activation. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Syrmanova K. K, Suleimenova M. T, Kovaleva A. Y, Botabayev N. Y, Kaldybekova Z. B. Vermiculite Absorption Capacity Increasingby Acid Activation. Orient J Chem 2017;33(1).Available from: http://www.orientjchem.org/?p=28067 |
Introduction
Vermiculite is a naturally occurring mineral that expands when heated.High adsorption and ion exchange properties, ease of obtaining modified, including organic-mineral forms, relatively good acid and alkali resistance determine widespread use and sustained interest in the research of structure and properties of vermiculite based material [1-3].
Vermiculite is a very valuable natural material. Expanded vermiculite, i.e. subjected to heat treatment is applied with a large economic impact in various sectors.Expanded vermiculite does not burn, is chemically inert, durable, biostable, completely explosion and fire safe, ecological, has a beautiful golden color, has ion exchange and sorption properties, a high capacity for absorbing and hold liquids and gases [4].
World reserves of vermiculite are large enough. There are deposits of vermiculite in the Republic of Kazakhstan. The need in vermiculite could reach tens of thousands of tons per year, thanks to a wide range of applications and the appearance of new areas of vermiculite application in various industries [5].
Improving the structural-sorption and strength characteristics, increasing the selectivity to certain substances, expanding the range, simplify production technology and reduce the cost of production of industrial adsorbents are urgent tasks facing science. Vermiculiteusing will allow the development of clean and efficient technologies for industrial wastewater treatment, oil, oil products and other chemical substances.
Material and Method
Vermiculite of Kulantau field in Kazakhstan was used as an object of the research. The standard methods of the research were used for the chemical analyzes of vermiculite samples and research of natural and expanded vermiculite diffraction pattern [6-7].
X-ray analysis of vermiculite took place on DRON-3 apparatus. Structural analysis and identification layer-crystal phases based on them are inherent values of the interlinear distance and intensity of X-ray spectral lines were carried out on polycrystalline samples. Vermiculite activation was performed with 25% H2SO4during 6 hours at temperature 95ºC and the ratio of the solid and liquid phases of S:L = 1:5.
Results and Discussion
It is known that the treatment of clay minerals by hot acids leads to inflation of their catalytic and adsorptive capacity [8-9]. In this regard, the research of vermiculite acid activation was carried out. Since the question of acids interaction with clay minerals remains largely unclear, further research is required to solve such an important theoretical and practical problems.
In the article the influence of treatment by hot sulfuric acid on the structure and adsorptive properties of vermiculite is considered. Vermiculite was activated by 25% concentrated sulfuric acid H2SO4 during 6 hours with the temperature 95ºC ratio of solid and liquid phases S:L = 1:5. Also influence of treatment by 5, 10, 15, 20, 25% concentrated sulfuric acid H2SO4 with ration S:L = 1:10 and under the same conditions was considered.
The results of chemical analysis (table 1, figure 1) show that during the activation occurs dissolving a considerable part of magnesium, iron, aluminum oxides, and it is the reason why the content of SiO2 is increased in samples.
Table 1: Chemical compound of initial and treated samples of vermiculite by 25% H2SO4 with S:L = 1:5
|
Components, % |
Initial samples |
Treated samples, % |
| Fe2O3 |
6,01 |
0,87 |
| FeO |
0,88 |
0,38 |
| SiO2 |
37,44 |
71,65 |
| MgO |
23,88 |
4,87 |
| Al2O3 |
11,23 |
2,04 |
| CaO |
2,10 |
1,05 |
| H2O |
10,98 |
17,8 |
| K2O + Na2O |
1,18 |
0,96 |
|
Others |
6,30 |
0,38 |
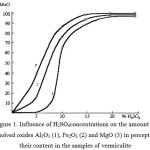 |
Figure 1: Influence of H2SO4concentrations on the amount of dissolved oxides Al2O3 (1), Fe2O3 (2) and MgO (3) in percents to their content in the samples of vermiculite |
Vermiculite is characterized by the largest relative increase in the silica content in the activation of from 37.44 to 71.65%. The quantity ofMgO in the activated samples by 25% H2SO4in comparison with initial samples decreases from 23.88 to 4.87%. Taking into account that approximately 18-20 weight parts of activated vermiculite is a source and adsorbed water, it can be concluded that the vermiculite activation leads to the production of almost pure silica.
X-Ray analysis results (figure 2 and figure 3) show that after activation of vermiculite diffraction peak peculiar to it – 14.2; 7.2; 4.7; 3.6; 2.9 A and others disappear and in their place in the diffraction pattern appears wide band centered 3.9 A, which once again demonstrates the complete amorphization of vermiculite. On the basis of the research results could be developed a method of technical amorphous silica (SiO2) obtaining by treatment with hot mineral vermiculite acid [9-10].
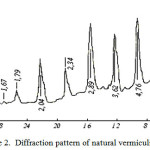 |
Figure 2: Diffraction pattern of natural vermiculite |
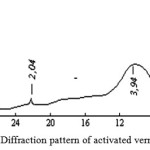 |
Figure 3: Diffraction pattern of activated vermiculite |
Table 2: Influence of activating acid concentration on chemical compound of vermiculite
| Sample |
H2SO4, % |
Components, % |
Total, % |
||||||||
|
SiO2 |
Al2O3 |
Fe2O3 |
FeO |
MgO |
CaO |
Na2O + K2O |
Н2О |
П.п.п. |
|||
| Vermiculite |
0 |
37,44 |
11,23 |
6,01 |
0,88 |
23,88 |
2,10 |
1,18 |
10,98 |
6,3 |
100 |
|
5 |
42,09 |
10,40 |
5,90 |
0,75 |
22,11 |
1,83 |
1,13 |
14,42 |
1,37 |
100 |
|
|
10 |
52,43 |
8,08 |
3,62 |
0,63 |
15,33 |
1,76 |
1,07 |
15,63 |
1,45 |
100 |
|
|
15 |
63,06 |
5,36 |
1,02 |
0,54 |
10,44 |
1,50 |
0,99 |
16,07 |
1,02 |
100 |
|
|
20 |
70,01 |
3,31 |
0,94 |
0,41 |
5,01 |
1,32 |
0,98 |
17,31 |
1,27 |
100 |
|
|
25 |
71,65 |
2,04 |
0,87 |
0,38 |
4,87 |
1,05 |
0,96 |
17,81 |
0,38 |
100 |
|
In the conditions of water adsorption vermiculite should be considered as a finely porous adsorbent with varying pore size. During sulfuric acid activation finely porous adsorptive vermiculite structure completely or partially disappears and forms an adsorbent with larger pores.
On the bases of experimental results on activation of vermiculite by hot solution of sulfuric acid some regularities were defined. The effectiveness of vermiculite activation depends primarily on the crystal structurecharacteristics. Data of a chemical analysis show that the vermiculite, which is characterized by a developed isomorphism in tetrahedral and octahedral positions strongly destroyed by treatment with sulfuric acid.This also contributes to the presence of octahedral and tetrahedral defect that facilitate penetration of the protons in the structure and contribute to the dissolution of magnesium oxide.
Thus, to create sorbents with advanced porosity by acid activation the choice of minerals with expanding structural unit is preferred. In the formation of advanced porosity sorbent the creation of thin layers of amorphous phase between poly- and partially destroyed by the elements of the original structure of vermiculite is of great importance. This leads to a disordered vermiculite sandwich packets mixing relative to each other and creates a more open pore system.
Under identical conditions of the activation the destruction of the expanding vermiculite structural unit initiates the formation of octahedral cations Fe3+, especially Mg2+ in their lattice.
Bonds Mg – O and Fe – O are less stable than Al – O, and the respective oxides are washed away in the first turn under the action to vermiculite by sulfuric acid.This explains the total disruption oftrioctahedral vermiculite during the activation by 25% sulfuric acid.
To evaluate the hydrophilicity of the surface of vermiculite its specific heat of wetting was determined. Due to the fact that a microporous adsorbent vermiculite, determining the specific surface area is difficult and therefore for an assessment of the hydrophilicity of vermiculite the ratio to the amount of wetting heat toamount of bound water was used.The effect of exchangeable cationsof acid treatment on the structure and adsorption properties of vermiculite was researched. It has been shown that replacement of vermiculite natural exchange complex to organic or inorganic cations leads to an appreciable increase in the adsorption capacity of vermiculite with respect to hydrocarbons.
Conclusion
Thus, the research results show that acidic treatment by hot acid leads to a extremal increase in vermiculite specific surface area and transition pores volume. At the same time there is the fact of preferential dissolution of magnesium and iron oxides. By the methods of X-ray analysis confirmed the formation of amorphous silica during the vermiculite treatment with hot sulfuric acid. It is shown that vermiculite can be used to produce technical silica, sincein the time of activation by 20-25% solution of sulfuric acid occurs total destruction of its grid and the formation of substantially pure silica.
The reasons for an anomalous hysteresis loops formation on the adsorption-desorption isotherms of polar substances by vermiculite, it could be explained by the introduction of adsorbed molecules in the inter-packet space of vermiculite and hydration (solvation) of exchangeable cations. Processing of water adsorption isotherms by vermiculite in Langmuir coordinate allows to obtain the value of adsorption corresponding to successive stages of hydration vermiculite.
References
- Gelperin N.I. Basic processes and apparatuses of chemical technology.Moscow.2011, 412
- Kasatkin A.G. Basic processes and apparatuses of chemical technology. Moscow.2003, 752
- GregS.; SingK. Adsorption, surface, porocity. Moscow.2010, 407
- Kalyanov N.N. Vermiculite, its properties, technology and application.Perlite and Vermiculite.2004, 110-123
- Iskritski N.A. Economics and perspectives of vermiculiteusing. Science Leningrad Addition.2005, 152
- Gorelik S.S.;RastorguyevL.N.;SkakovU.A. X-ray and electron-optical analysis.Moscow.2000,252
- Braun M. X-ray methods of studying the structure of clay minerals. Moscow.2005,599
- AivazovB.V. Surface chemistry and adsorption.Moscow.2003,295
- Syrmanova K.K.;KaldybekovaZh.B.Poly functional sorbents.Shymkent.2012,168

This work is licensed under a Creative Commons Attribution 4.0 International License.









