Synthesis and Biological Activity of Novel (E)-N’-(Substituted)-3,4,5-Trimethoxybenzohydrazide Analogs
Namala Rambabu*1,3, Bhavani Ram3,Pramod Kumar Dubey1, Bhavani Vasudha2 and Bhavani Balram3
1Department of Chemistry, Jawaharlal Nehru Technological University, Hyderabad-500 072, Telangana State, India.
2Department of Pharmacology, Lalitha College of Pharmacy, Ghatkesar, 500088, Telangana State, India.
3Green Evolution Laboratories, Wangapally Village, Nalgonda- 500085, Telangana State, India.
Corresponding Author E-mail: rambabun2013@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330126
The hydrazide-hydrazone analogs 4a-4l is described via the condensation of3,4,5-trimethoxybenzohydrazide 3 with various aromatic and hetero aromatic aldehydes a-l. Various spectroscopic techniques viz., (1H NMR, 13C NMR, IR and MS) were utilized to determine the structures of synthesized compounds. These compounds were evaluated for antibacterial, antifungal screening against S.aureus, S.pyogenes, E.coli, P.aeruginosa, Aspergillusnigerand Candida albicans(Fungal strains). The results revealed that most of the hydrazone derivatives exhibited significant antibacterial activity. Furthermore, the synthesized hydrazone derivatives were found to exhibit significant antidiabetic activity when compared to insulin.
KEYWORDS:Eudesmic acid; Hydrazones; Gallic acid; Antibacterial activity; Synthesis
Download this article as:| Copy the following to cite this article: Rambabu N, Ram B, Dubey P. K, Vasudha B, Balram B. Synthesis and Biological Activity of Novel (E)-N’-(Substituted)-3,4,5-Trimethoxybenzohydrazide Analogs. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Rambabu N, Ram B, Dubey P. K, Vasudha B, Balram B. Synthesis and Biological Activity of Novel (E)-N’-(Substituted)-3,4,5-Trimethoxybenzohydrazide Analogs. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=28393 |
Introduction
Eudesmic acid is a o-methylated analogue of gallicacid and is found in Eucalpytusspp1. The chemical name of gallicacid, is 3,4,5-trihydroxybenzoic acid, and exists either in the pure form or as a phenoilic constituent in various parts of the plant morphology such as roots, leaves, bark and fruits 2. 3,4,5-trihydroxybenzoic acid possess analgesic activity3,anti-inflammatory4,hepatoprotective5, anti-tumor potentials 6, antimutagenic7,neuroprotective activity8, and toxicity against cancer cells9. 3,4,5-trihydoxybenzoic acid (gallic acid) and its associated analogs are used in the following applications such as (a) photographic developer, (b) testing of free mineral acids, (c) di-hydroxy acetone and alkaloids10. Furthermore, in cases of internal hemorrhoids these are known as (d) a styptic agent (e) an astringen.
Hydrazones are a category of organic compounds that possess the structure R1R2C=NNH2. They are associated to ketones and aldehydes in which oxygen atom has been replaced with -NNH2 moiety. This azomethine –NHN=CH–protons represent a very important category of compounds for drug development. Hydrazones are prepared by the reaction of hydrazide with aldehydes and ketones. Hydrazones and its derivatives exhibit various pharmacological activities like analgesic, antiplatelet, antiprotozoal, antimicrobial, anticonvulsant, analgesic, antimycobacterial, anticancer, vasodilator, antiviral, anti-HIV, anthelmintic, and trypanocidal activities 11-19. Inspired by the various pharmacological and medicinal importance connected with gallic acid and hydrazone moiety, we wish to report the synthesis, antibacterial, antifungal, antidiabetic activity of the synthesized hydrazone derivatives 4a-l from commercially available 3,4,5-trihydroxy benzoic acid (gallic acid,).
Results and Discussion
Chemistry
The synthetic sequence of hydrazone derivatives 4a-l derived from gallic acid is illustrated in scheme 1. Methylation of gallic acid with dimethyl sulphate in the presence of aq.10% NaOH resulted in eudesmic acid (3,4,5-trimethoxy benzoicacid) 2. Preparation of 3, 4, 5-trimethoxybenzohydrazide 3 was achieved by two methods, Method 1: esterification of eudesmic acid (3,4,5-trimethoxy benzoicacid) 2 in presence of amberlyst-15 in MeOH20followed by reaction with hydrazine hydrate gave 3, 4, 5-trimethoxybenzohydrazide3 in 75% yield. Method 2: Reaction of 2 with hydrazine hydrate in presence of HATU and DIPEA in THF resulted in 3,4,5-trimethoxybenzohydrazide3 in 95% yield. Condensation of 3, 4, 5-trimethoxybenzohydrazide3 with various aldehydes a-l was carried out in presence of refluxing ethanol for 1h to result in hydrazone derivatives 4a-l in quantitative yields. Structural elucidations of the synthesized hydrazones were established by IR, mass and 1H NMR analysis. (i) 1H NMR interpretation of (E)-3,4,5-trimethoxy-N’-((5-nitrothiophen-2-yl)methylene)benzohydrazide4h: the singlet signals at 12.02 ppm, 8.73 ppm and 7.22 ppm correspond to –CO-NH-, –HC=N- and phenyl ring respectively. The signal with doublet pattern appearing at 8.12 ppm (1H) and 7.56 ppm (1H) corresponds to thiophene ring protons, while the signals at 3.74 ppm ( 3H) and 3.85 ppm ( 6H) represents the methoxy groups. Similarly, the proton NMR signal of the remaninghydrazone derivatives is in agreement with the desired structures. (ii) IR spectra of the compounds 4a-l: Peaks in the region 1644-1646 cm-1, 1406-1615 cm-1, 1225-1335 cm ,and 3427-3486 cm-1 is assigned to the following functional groups viz., -C=O of amide functional groups,- C=N, -aromatic ring, -C-N and –NH stretching vibrations respectively. (iii) Mass spectra of the compounds 4a-l: (M+H)+ of all the hydrazones are in agreement with their molecular formulae.
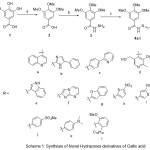 |
Scheme 1: Synthesis of Novel Hydrazones derivatives 4a-l Reaction conditions: a) DMS, aq.10% NaOH, R.T., 10 h; b) Method-1: i. Amberlyst-15, methanol, reflux, 24 h; ii. NH2-NH2.H2O (99% w/v), reflux, 10 h; Method-2: HATU, DIPEA, NH2-NH2 (100%), R.T., 6 h; c) Aldehydes a-l, EtOH, reflux, 1h Click here to View scheme |
Biological Evaluation
Antimicrobial activity
The zone of inhibition (ZI) values of hydrazone derivatives 4a-l is complied in Table 1. The results revealed that, compounds 4a-f showed excellent antimicrobial activity with ZI (15-21 mm) whereas compounds 4g, 4h, 4i and 4l showed good activity with ZI (12-15 mm) and the remaining two compounds 4j and 4k displayed moderate activity with ZI (8- 10 mm) against S.aureus and S.pyogenes. Compounds 4j and 4k displayed moderate activity with ZI (10- 12 mm), compounds, 4g-i and 4l showed good activity with ZI (11-14 mm) while the compounds 4a, 4b, 4c, 4d, 4e, and 4f exhibited excellent activity with ZI (17-20 mm) against E.coli and P.aeruginosa. A careful observation from the ZI values indicated that within the series of hydrazones derivatives compounds 4f, 4b and 4d showed highest ZI values compared to the standard drug ampicillin (ZI = 14-16 mm). In case of Aspergillusniger(MTCC 282) and Candida albicans(MTCC 227), compounds 4a-f showed moderate antifungal activity with ZI (10-14 mm) when compared to the standard Greseofulvin (ZI = 22 – 25 mm). It’s noteworthy to mention that hydrazone derivatives 4a-l showed significant antibacterial inhibition in comparison to the fungal activity.
Table 1.Antimicrobial screening results of hydrazid-hydrazone derivatives 4a-l
|
Compound |
Gram-positive bacterial strains |
Gram-negative bacterial strains |
Fungal strains |
|||
|
S.a
|
S.p
|
E. c
|
P. a
|
A.n
|
C.a
|
|
|
Diameter of Zone of inhibition in mm |
||||||
|
4a |
16(±0.78) |
18(±0.58) |
17(±0.62) |
17(±0.57) |
11(±0.41) |
10(±0.38) |
|
4b |
17(±0.92) |
21(±0.76) |
20(±0.76) |
19(±0.78) |
13(±0.38) |
9(±0.51) |
|
4c |
15(±0.68) |
17(±0.88) |
18(±0.73) |
18(±0.58) |
10(±0.44) |
8(±0.67) |
|
4d |
16(±0.58) |
18(±0.48) |
18(±0.78) |
19(±0.72) |
14(±0.71) |
11(±0.61) |
|
4e |
15(±0.87) |
17(±0.59) |
17(±0.88) |
17(±0.81) |
12(±0.68) |
10(±0.78) |
|
4f |
18(±0.94) |
20(±0.62) |
19(±0.58) |
20(±0.69) |
14(±0.65) |
12(±0.82) |
|
4g |
13(±0.43) |
15(±0.67) |
14(±0.47) |
14(±0.72) |
– |
– |
|
4h |
13(±0.55) |
13(±0.88) |
14(±0.65) |
13(±0.58) |
– |
– |
|
4i |
12(±0.91) |
13(±0.72) |
13(±0.54) |
13(±0.49) |
– |
– |
|
4j |
8(±0.44) |
10(±0.51) |
11(±0.61) |
12(±0.39) |
– |
– |
|
4k |
9(±0.68) |
10(±0.48) |
10(±0.71) |
11(±0.82) |
– |
– |
|
4l |
12(±0.81) |
14(±0.83) |
15(±0.81) |
11(±0.91) |
– |
– |
| Ampicillin a |
14(±0.2) |
16(±0.3) |
16(±0.1) |
15(±0.3) |
||
| Greseofulvinb |
25(±0.2) |
22(±0.2) |
||||
a: Concentration: 50 μg/mL-1); b: Concentration: 50 μg/mL; ‘-‘: No activity; A.n: Aspergillusniger(MTCC 282); C.a: Candida albicans(MTCC 227); E.c: Escherichia coli (MTCC 443); P.a: Pseudomonas auriginosa(MTCC 424); S.a: Staphylococcus aureus(MTCC 96); S.p: Streptococcus pyogenes(MTCC 442);
Antidiabetic Activity
Experimental in vivo studies of alloxan induced diabetic model in rat was carried for the all the synthesized hydrazone derivatives in order to understand the anti diabetic property. The results of the measurement of blood glucose (%) level are presented in Table 2. Though there is a certain varied difference in the blood glucose levels within the treatment of diabetic rats, it is evident from the table-2 that most of the hydrazone compounds exhibited significant anti diabetic property (a significant reduction in blood glucose) compared to control diabetic rats at 50 mg/kg body weight for 3rd and 7th days.
Table 2: Results of hypoglycemic effects of hydrazone derivatives 4a-l
|
Treatment (mg/kg b.wp.o) |
Blood glucose level (mg/dl) |
|||
|
Day-0 |
Day-3 |
Day-7 |
Hyperglycemic activity (%) |
|
|
Control (0.5% CMC) |
345.0 ± 2.48 |
376.3 ± 3.54** |
395.1 ± 3.03** |
|
|
Insulin |
351 ± 3.98 |
140.60 ± 3.54** |
106.1 ± 3.56** |
69.77 |
|
4a |
338.4 ± 2.75 |
179.30 ± 1.82** |
119.0 ± 3.66** |
64.83 |
|
4b |
322.5 ± 2.75 |
203.60 ± 3.41** |
117.3 ± 2.59** |
63.62 |
|
4c |
354.1 ± 2.37 |
258.50 ± 2.28** |
148.50 ± 3.31** |
58.06 |
|
4d |
344.6 ± 3.33 |
237.50 ± 2.06** |
110.44 ± 1.98** |
67.95 |
|
4e |
339.3 ± 3.39 |
236.10 ± 2.43** |
116.50 ± 3.99** |
65.66 |
|
4f |
338.6 ±2.40 |
220.30 ± 1.66** |
115.10 ±1.60** |
66.00 |
|
4g |
352.3 ± 3.37 |
273.3 ± 1.65** |
158.0 ± 3.69** |
55.15 |
|
4h |
344.3 ± 3.95 |
258.10 ± 3.63** |
155.60 ± 3.69** |
54.80 |
|
4i |
348.4 ± 2.77 |
289.60 ± 3.26** |
146.10 2.36** |
58.06 |
|
4j |
353.4 ± 3.46 |
277.30 ± 3.80** |
156.60 ± 1.92** |
55.68 |
|
4k |
346.5 ± 2.46 |
262.10 ± 2.26** |
157.40 ± 3.46** |
54.57 |
|
4l |
354.1 ± 3.46 |
243.30 ± 3.67** |
156.50 .5 ± 3.46** |
55.80 |
**P < 0.001; Tabulated data are expressed as mean ± SEM; (n=6)
For the antidiabetic study, insulin was taken as the standard drug at the dose of 50 mg/kg.p.o which showed 69.77% blood glucose lowering activity. Within the series of the synthesized hydrazone derivatives, compound 4d (67.95%)having R = 3-hydroxy-5-(hydroxymethyl)-2-methylpyridinexhibited significant antidiabetic activity when compared to insulin (50 mg/kg b.w) in dropping the blood glucose level. It is interesting to deduce that compounds 4f (66.0%) having R = imidazo[1,2-a]pyrimidin-2-yl)methylene, 4e (65.66%) having R = 1H-indol-3-yl)methylene, 4a (64.83%) having R = quinolin-4-yl)methylene) and 4b (63.62%) having R = 4-phenyl-1H-imidazol-2-yl)methyl displayed an substantial antidiabetic activity (increase in hypoglycemic activity). Compound 4c (58.06%) and compound 4i (58.06%) relatively had shown moderate hypoglycemic activity whereas the compounds 4l, 4j, 4g, 4h, and 4k show 55.80%, 55.68%, 55.15, 54.80 and 54.57% poor hypoglycemic property respectively. In general, it is significant to observe that hydrazone derivatives embedded with nitrogen-containing heterocycles exhibited vital hypoglycemic activity and the rest of the compounds in the series displayed modest to poor hypoglycemic activity. From the ascertained result, it is concluded that compounds 4a, 4b, 4d, 4e, and 4f reduced the glucose level in diabetic rats. But the impact of 4d is more distinct in alloxan induced diabetic rats. Investigation into additional toxicity studies and understanding the mechanism of action of hypoglycemic activity will offer an efficient drug candidate for diabetes mellitus.
Experimental Section
Materials and Methods
All the chemical and solvents used for the synthesis were analytical standard from Fluka or Merck. For thin-layer chromatography (TLC) analysis, E.Merck AL silica gel 60 F254 plates were utilized and spots were visualized under UV light. The mass spectra was recorded on Agilent ion trap MS and Infrared (IR) spectra were recorded on a Perkin Elmer FT-IR spectrometer. 1H NMR and 13 C NMR spectra were recorded in DMSO- d6 with a 400 MHz (Varian Mercury plus) instrument. TMS was used as an internal standard and the chemical shift values were reported in δ (ppm) and the signals were reported as s (singlet), d (doublet), dd (doublet of doublet), t (triplet), q (quartet), m (multiplet) and coupling constants are measured in Hz. Melting point (mp) determinations were performed by using Mel-temp apparatus and are uncorrected. The aldehydes f and l 21, 22was synthesized as per the previous reported literature procedures.
3, 4, 5-Trimethoxybenzoic acid
Dimethyl sulphate (1.94g, 17.6 mmol) was added slowly over a period of 10 min to a cooled solution of compound 1(1g, 5.88 mmol) in 10% NaOH sol (20 mL ) maintained at 10-15oC. The reaction mixture was allowed to attain room temperature and stirred for 10 h. The completion of the reaction was checked by TLC followed by acidification by 6N HCl (25 mL) and then filtered to obtain compound 2. Brown solid; Yield: 74%; M.p.: 80-82°C; 1H NMR (400 MHz, DMSO-d6): δ 3.72 (s, 3H), 3.85 (s, 6H), 7.23 (s, 1H), 12.90 (brs, 1H).
3, 4, 5-trimethoxybenzohydrazide
Method 1: A mixture of 3, 4, 5-Trimethoxybenzoic acid2 (1g, 4.71 mmol) and Amberlyst -15 (300 mg) in methanol (20 mL) was refluxed for 24 h in a pressure tube. T.L.C of the reaction indicated absence of starting material; the insoluble Amberlyst-15 was filtered at the pump and rinsed with methanol (4 X 2 mL). The combined methanol filtrates (containing methyl 3, 4, 5-trimethoxybenzoate) was further reacted with hydrazine hydrate (1.09 mL, 21.8 mmol) and heated at its reflux temperature for 10 h. The reaction mixture was concentrated to one-fourth volume and cooled to 5oC to isolate compound 4 (3, 4, 5-trimethoxybenzohydrazide) as a white solid. Yield: 74%; M.p.: 156-158°C; 1H NMR (400 MHz, DMSO-d6): δ 3.74 (s, 3H), 3.84 (s, 6H), 4.45 (brs, 2H), 7.15 (s, 2H), 9.70 (brs, 1H);MS (ESI) m/z: 227.20 [M+H]+.
Method 2: Compound 2(1g, 4.71 mmol) was dissolved in THF (10 mL) containing DIPEA (5.65 mmol) and cooled to 5°C. To the above solution, HATU (1.79 g, 4.71 mmol) was added in five portions and stirred at room temperature for 4 h. Water (20 mL) was added to the reaction contents and was taken in dichloromethane (50 mL), the organic layer was separated, washed with water (2 X 15 mL) and then with brine solution. The dichloromethane layer was dried over Na2SO4, filtered and concentrated under reduced pressure to isolate compound 3. Yield: 95%.
Preparation of Hydrazone derivatives 4a-l
Aromatic/heteroaromatic aldehydes a-l (1.0 mmol) was added to ethanol (2 mL) containing compound 3 (100 mg, 0.40 mmol) and heated to reflux point for 1h. The solids obtained was filtered and slurred with ethanol (1mL) followed by n-Hexane to get the hydrazones4a-l .
(E)- 3,4,5-trimethoxy-N’-((quinolin-4-yl)methylene)benzohydrazide 4a
White solid; Yield: 94%; M.p.; 124-125°C; IR (KBr): υmax cm-13433, 3211, 3065, 3032, 3002, 2948, 2838, 1644, 1583, 1540, 1502, 1459, 1414, 1391, 1367, 1354, 1335, 1269, 1247, 1238, 1190, 1181, 1133, 1072, 1015, 1000, 974, 864, 850, 815, 768, 755, 737, 703, 671, 622; 1H NMR (400 MHz, DMSO-d6): δ 3.75 (s, 3H), 3.89 (s, 6H), 7.30 (s, 2H), 7.75 (d, J = 7.2 Hz, 1H), 7.83 (d, J = 8.4 Hz, 1H), 8.10 (d, J = 8.4 Hz, 1H), 8.79 (d, J = 8.4 Hz, 1H), 9.00 ( d, J = 4.4 Hz, 1H), 9.09 (s, 1H), 12.05 (s, 1H); MS (ESI) m/z: 366.3 [M+H]+.
(E)-3,4,5-trimethoxy-N’-((4-phenyl-1H-imidazol-2-yl)methyl)benzohydrazide 4b
White solid; Yield: 92%; M.p.: 137-138°C; IR (KBr): υmax cm-13497, 3398, 3206, 3116, 3038, 3012, 2954, 2937, 2840, 2634, 1649, 1607, 1584, 1519, 1504, 1465, 1414, 1361, 1330, 1305, 1276, 1247, 1239, 1221, 1187, 1175, 1127, 1062, 1007, 917, 856, 826, 809, 769, 737, 663, 621, 548; 1H NMR (400 MHz, DMSO-d6): δ 3.73 (s, 3H), 3.87 (s, 6H), 7.15 (brs, 1H), 7.24 (s, 2H), 7.77 ( d, J = 8.0 Hz, 2H), 7.84-7.82 (m, 2H), 8.36 (brs, 1H), 8.51 (s, 1H), 11.8 (s, 1H); MS (ESI) m/z: 381.3 [M+H]+.
(E)-N’-(4-(pyridine-2-yl)benzylidene)-3,4,5-trimethoxybenzohydrazide 4c
White solid; Yield: 86%; M.p.: 132-133°C; IR (KBr): υmax cm-13445, 3212, 3052, 3005, 2977, 2935, 2871, 2836, 1731, 1639, 1584, 1537, 1502, 1465, 1435, 1415, 1360, 1331, 1269, 1236, 1180, 1129, 1066, 1048, 994, 941, 846, 814, 783, 768, 740, 722, 672, 639, 616; 1H NMR (400 MHz, DMSO-d6): δ 3.74 (s, 3H), 3.87 (s, 6H), 7.25 (s, 2H), 7.37 (dd, J = 4.8, 6.0 Hz, 1H), 7.85 (d, J = 7.6 Hz, 2H), 7.89-7.93 (m, 1H), 8.0 (d, J = 8.0 Hz, 1H), 8.19 (d, J = 8.4 Hz, 2H), 8.52 (s, 1H), 8.69 (d, J = 4.0 Hz, 1H), 11.80 (s, 1H); MS (ESI) m/z: 392.3 [M+H]+.
(E)-N’-((3-hydroxy-5-(hydroxymethyl)-2-methylpyridin-4-yl)methylene)-3,4,5-trimethoxybenzohydrazide 4d
Yellow solid; Yield: 82%; M.p.: 123-124 °C; IR (KBr): υmax cm-13486, 3186, 2942, 2840, 1650, 1586, 1556, 1502, 1462, 1416, 1394, 1329, 1265, 1225, 1182, 1127, 1067, 1028, 1003, 962, 867, 817, 766, 728, 696, 667; 1H NMR (400 MHz, DMSO-d6): δ 2.50 (s, 3H), 3.75 (s, 3H), 3.88 (s, 6H), 4.62 (d, J = 4.4 Hz, 2H), 5.42 (t, J = 3.6 Hz, 1H), 7.32 (s, 2H), 7.96 (s, 1H), 8.94 (s, 1H), 12.30 (s, 1H), 12.45 (s, 1H); MS (ESI) m/z: 374.0 [M+H]+.
(E)-N’-((1H-indol-3-yl)methylene)-3,4,5-trimethoxybenzohydrazide 4e
Pale yellow solid; Yield: 86%; M.p.: 132-133°C; IR (KBr): υmax cm-13334, 3050, 3013, 2989, 2966, 2940, 2885, 2835, 1949, 1635, 1608, 1586, 1522, 1503, 1465, 1456, 1425, 1415, 1366, 1343, 1247, 1238, 1175, 1128, 1105, 1058, 1010, 989, 969, 859, 821, 765, 748, 610, 598, 544, 528; 1H NMR (400 MHz, DMSO-d6): δ 11.53 (s, 1H), 11.32 (s, 1H), 8.62 (s, 1H), 8.28 (d, J = 7.6 Hz, 1H), 7.80 (s, 1H), 7.43 (d, J = 8.0 Hz, 1H), 7.24-7.12 (m, 4H), 3.86 (s, 6H), 3.78 (s, 3H); MS (ESI) m/z: 354.3 [M+H]+.
(E)-N’-((imidazo[1,2-a]pyrimidin-2-yl)methylene)-3,4,5-trimethoxybenzohydrazide 4f
Yellow solid; Yield: 72%; M.p.:129-130°C; IR (KBr): υmax cm-13486, 3186, 2942, 2840, 1650, 1586, 1556, 1502, 1462, 1416, 1394, 1329, 1265, 1225, 1182, 1127, 1067, 1028, 1003, 962, 867, 817, 766, 728, 696, 667; 1 H NMR (400 MHz, DMSO-d6): δ 11.85 (s, 1H), 9.85 (d, J = 6.0 Hz, 1H), 8.8 (s, 2H), 8.25 (s, 1H), 7.40 (m, 1H), 7.20 (s, 2H), 3.92 (s, 6H), 3.80 (s, 3H); MS (ESI) m/z: 356.40 [M+H]+.
(E)-N’-((benzofuran-2-yl)methylene)-3,4,5-trimethoxybenzohydrazide 4g
Yellow solid;Yield: 86%; M.p.: 97-98 °C; IR (KBr): υmax cm-13437, 3192, 3164, 3103, 3019, 2996, 2966, 2954, 2935, 2875, 2834, 2822, 1950, 1646, 1615, 1586, 1568, 1537, 1500, 1463, 1449, 1428, 1413, 1345, 1325, 1292, 1260, 1240, 1228, 1187, 1174, 1142, 1116, 1083, 998, 988, 951, 942, 885, 862, 850, 814, 801, 779, 753, 725, 670, 613; 1H NMR (400 MHz, DMSO-d6): δ 3.74 (s, 3H), 3.88 (s, 6H), 7.25 (s, 2H), 7.28 (t, J = 7.2 Hz, 1H), 7.36 (s, 1H), 7.40 (t, J = 7.2 Hz, 1H), 7.66 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 7.6 Hz, 1H), 8.50 (brs, 1H), 11.82 (brs, 1H); MS (ESI) m/z: 355.1 [M+H]+.
(E)-3,4,5-trimethoxy-N’-((5-nitrothiophen-2-yl)methylene)benzohydrazide 4h
Yellow solid; Yield: 86%; M.p.: 112-113 °C; IR (KBr): υmax cm-13437, 3211, 3106, 3082, 3020, 2958, 2939, 2838, 1643, 1586, 1541, 1499, 1462, 1455, 1413, 1337, 1321, 1265, 1239, 1196, 1189, 1166, 1133, 1086, 1075, 996, 957, 929, 865, 844, 810, 771, 752, 738, 729, 684, 668, 613, 530; 1H NMR (400 MHz, DMSO-d6): δ 3.74 (s, 3H), 3.85 (s, 6H), 7.22 (s, 2H), 7.56 (d, J = 4.4 Hz, 1H), 8.12 (d, J = 4.0 Hz, 1H), 8.73 (brs, 1H), 12.02 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 162.7, 152.7, 151.9, 141.6, 136.5, 133.8, 128.2 (3C), 126.6, 105.3 (2C), 60.1, 56.1 (2C); MS (ESI) m/z: 366.26 [M+H]+.
(E)-3,4,5-trimethoxy-N’-((5-nitrothiophen-3-yl)methylene)benzohydrazide 4i
Yellow solid; Yield: 86%; M.p.: 119-120 °C; IR (KBr): υmax cm-13437, 3211, 3106, 3082, 3020, 2958, 2939, 2838, 1643, 1586, 1541, 1499, 1462, 1455, 1413, 1337, 1321, 1265, 1239, 1196, 1189, 1166, 1133, 1086, 1075, 996, 957, 929, 865, 844, 810, 771, 752, 738, 729, 684, 668, 613, 530; 1H NMR (400 MHz, DMSO-d6): δ 3.73 (s, 3H), 3.85 (s, 6H), 7.22 (s, 2H), 8.28 (s, 1H), 8.32 (s, 1H), 8.46 (brs, 1H), 11.78 (s, 1H); MS (ESI) m/z: 366.27 [M+H]+.
(E)-N’-(4-(methylsulfonyl)benzylidene)-3,4,5-trimethoxybenzohydrazide 4j
White solid; Yield: 86%; M.p.: 137-138 °C; IR (KBr): υmax cm-13443, 3170, 3067, 3005, 2983, 2941, 2925, 2889, 2836, 2631, 1955, 1701, 1638, 1583, 1560, 1503, 1463, 1454, 1411, 1367, 1337, 1313, 1292, 1277, 1235, 1198, 1181, 1169, 1153, 1130, 1083, 1069, 993, 969, 851, 839, 820, 778, 767, 745, 729, 696, 666, 567, 544, 530, 486; 1H NMR (400 MHz, DMSO-d6): δ 3.30 (s, 3H), 3.74 (s, 3H), 3.87 (s, 6H), 7.25 (s, 2H), 8.00 (s, 4H), 8.55 (s, 1H), 11.92 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 162.7, 152.7, 145.7, 145.2, 141.4, 140.6, 139.2, 139.0, 130.2, 128.2, 127.7 (3C), 105.3, 60.1, 56.1, 43.4, 43.1;MS (ESI) m/z: 390.9 [M+H]+
(E)-N’-(4-(dimethylamino)benzylidene)-3,4,5-trimethoxybenzohydrazide 4k
White solid; Yield: 82%; M.p.: 123-124 °C; IR (KBr): υmax cm-13444, 3205, 3077, 3035, 2994, 2963, 2936, 2838, 2632, 2103, 1636, 1610, 1593, 1583, 1566, 1552, 1524, 1500, 1463, 1448, 1413, 1362, 1331, 1285, 1229, 1182, 1170, 1123, 1103, 1060, 1001, 971, 944, 858, 717, 761, 738, 630, 526, 494; 1H NMR (400 MHz, DMSO-d6): δ 2.98 (s, 6H), 3.72 (s, 3H), 3.86 (s, 6H), 6.76 (d, J = 7.2 Hz, 2H), 7.21 (s, 2H), 7.54 (d, J = 6.8 Hz, 2H), 8.32 (s, 1H), 11.39 (s, 1H); 13C NMR (100 MHz, DMSO-d6) δ: 162.0, 152.6 (2C), 151.5 (2C), 148.6, 128.9, 128.3 (3C), 121.5, 111.8, 105.0 (2C), 60.0, 56.0(3C), 40.1, 39.9;MS (ESI) m/z: 358.1 [M+H]+.
(E)-N’-(2-methoxy-6-pentadecylbenzylidene)-3,4,5-trimethoxybenzohydrazide 4l
Off white solid; Yield: 86%; M.p.: 132-133 °C; IR (KBr): υmax cm-13427, 3276, 3158, 3071, 2957, 2919, 2848, 1646, 1590, 1514, 1474, 1406, 1372, 1301, 1254, 1179, 1152, 1071, 1010, 973, 836, 813, 770, 734, 724, 672, 653, 593, 570, 478; 1H NMR (400 MHz, DMSO-d6): δ 11.63 (s, 1H), 8.77 (s, 1H), 7.27 (s, 3H), 6.92 (d, J = 7.2 Hz, 1H), 6.85 (d, J = 5.6 Hz, 1H), 3.86 (s, 9H), 3.73 (s, 3H), 2.97 (brs, 2H), 1.52 (brs, 2H), 1.34 (brs, 2H), 1.18 (m, 22H), 0.85 (s, 3H); MS (ESI) m/z: 555.1 [M+H]+.
Biology
Antimicrobial activity test
The antibacterial and antifungal screening test of hydrazones4a-l was done as per the standard agar diffusion method23. The microbial strains utilized for the study are (a) Aspergillusniger(MTCC 282), Candida albicans(MTCC 227) categorized under fungal strain (b) Escherichia coli (MTCC 443), Pseudomonas auriginosa(MTCC 424), Staphylococcus aureus(MTCC 96), Streptococcus pyogenes (MTCC 442) categorized under bacterial strains. Ampicillin(for antibacterial study) and Greseofulvin (for fungal study) are used as the standard reference drugs respectively under alike conditions for assessment. 1% Dimethyl sulphoxide was used as a blank reading. The MIC (minimum inhibitory concentration) was found at 50 µg/mL (using dimethyl sulfoxide as solvent) for the both fungal and bacterial study. The culture strains of fungi was maintained on potato dextrose agar (PDA) slant at 27±0.2°C for 24-48 hrs (till sporulation) while the bacteria was maintained on nutrient agar slant at 37±0.5°C for 24 h. The antimicrobial activity was assessed as follows (i) in case of antibacterial study: nutrient agar plate seeded with 0.1 mL of the respective bacterial culture strain suspension prepared in sterile saline (0.85%) of 105 CFU/mL dilutions (ii) in case of antifungal study: Sterile PDA plate was prepared containing 2% agar; 0.1 mL of each fungal spore suspension was spread on each plate and incubated at 27±0.2 °C for 12 hrs. Target compounds viz., synthesized hydrazone derivatives (0.1 mL), dilution ranging from 25 to 1000 μg/mL was poured into wells (6 mm diameter) separately for individual bacteria and fungi strains. All the plates were incubated at 37±0.5°C for 24 h (for bacterial study) and 27±0.2 °C for 12 hrs (for fungal study) and respective MIC values were determined for all the compounds.
By measuring the diameter of the inhibition zone around the well (in mm, including the well diameter), the zone of inhibition was calculated. The readings were recorded three times and the average values were tabulated (data are expressed in the form of mean of inhibition zone diameter for test compound performed in triplicate ±SD).
Pharmacological evaluation
Acute toxicity studies
Assembly of six mice (albino, weighing ~20–25 g) were fasted all night and administered orally with the test compounds 24, 25. The dose of the compounds was assorted from 500 mg kg−1 body weight. Signs of acute toxicity viz., motor activity, lacrimation, sedation, tremors, convulsion was observed for the mice for 24 h and the test results revealed that no mortality of the mice was observed even beyond 24 h. Based on the above observation, the test compound’s cut-off value, LD50 was fixed as 50 mg kg−1, consequently that 500 mg kg−1 i.e., 1/10 of cut-off value was taken as the dose quantity for assessment of antidiabetic activity. Animal experiments involved during the course of the study were undertaken by the approval of (a) Institutional Animal Ethics Committee, Anurag Group of Institutions (formerly Lalitha college of Pharmacy), Hyderabad, India and also (b) guidelines of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), IAEC ( Institutional Animals Ethics Committee) followed for the protection of animals. IAEC No:I/IAEC/LCP041/2014/SM40.
Antidiabetic activity
Alloxan induction of experimental diabetes
Overnight-fasted swiss albino mice are injected with alloxan (50mg/kg) in saline buffer intraperitoneally. After 72 hours, blood glucose levels were determined and mice having blood glucose levels <145mg/dl were excluded from experiment and rest were divided into ten groups. After 72 h of alloxan injection, confirmation of hyperglycemia was observed in animals.
Protocol for Animal Experiments
Animals were separated into ten sets of six mice (n = 6): (i) diabetic animals (vehicle) labeled as set-1, received 1m L of CMC (0.5%); (ii) diabetic animals labeled as set-2, received insulin 50 mg/Kg. (iii) Set (3–10) diabetic animals received compounds 4a–4l in a single dose (50 mg/kg body weight per oral) respectively for seven days incessantly
Measurement of Blood glucose levels
Each individual reading was noted by withdrawing blood sample from the tail vain. Measurement of blood glucose was taken at intervals of 0, 3rd and 7th day. By snipping the tip of the tail, blood samples were withdrawn from a tail vein and the blood glucose level was measured at the end of 0, 3rd and 7th day (by Accu Sure Blood Glucose Monitoring System, Dr. Gene Health & Wellness).
Computational analysis
The blood glucose level readings are presented as mean ± SEM and were determined using analysis of variance and group means were differentiated with Tukey–Kramer Post ANOVA test. The readings were considered when P <0.01.
Conclusion
The synthesis of somenovel (E)-N’-(substituted)-3,4,5-trimethoxybenzohydrazide derivatives is described from commercially available gallic acid. The newly synthesized hydrazone derivatives 4a-4l was evaluated for antimicrobial and antidiabetic activity. In case of bacterial and fungal studies, compounds 4a, 4b, 4c, 4d, 4e, and 4f exhibited excellent antibacterial activity and moderate antifungal activity. Hydrazone derivatives embedded with nitrogen-containing heterocycles exhibited vital hypoglycemic activity and the hydrazone compounds embedded with aromatic ring displayed modest to poor hypoglycemic activity.
Acknowledgements
Authors thank the management Lalithacollege of pharmacy for supporting the biological evaluation studies during the course of the work.
References
- Conde, E.; Cadahía, E.; Garcia-Vallejo, M.C.; Chromatographia. 1995, 41, 657.
CrossRef - Singleton, V.L. Adv Food Res.1981, 27, 149.
CrossRef - Krogh, R.; Yunes, R.Farmaco. 2000, 55, 730.
CrossRef - Stich, H.F.; Rosin, M.P.; Brison, L.Mutat Res.1982, 95, 119.
CrossRef - Anjana, J.; Monika, B.; Shukla, S. Journal of Ethnopharmacology.2007, 109, 214.
CrossRef - Chiara, D.; Giorgia, P.; Federica, M.; Gianni, Z.; Gaetano, B.; Silvia, M.; Gianluca, V. Cancer Letters.2005, 26, 17.
- Ohno, Y.; Fukuda, K.; Takemura, G.; Toyota, M.; Watanabe, M.; Yasuda, N.; Xinbin, Q.; Maruyama, R.; Akao, S.; Gotou, K.; Fujiwara, T.; Fujiwara, H. Anticancer Drugs.1999, 10, 845.
CrossRef - Zhongbing, N.; Guangjun, N.; Peter, S.; Belton, C.; Huiru, T. Neurochemistry International.2006, 48, 263.
CrossRef - Gali, H.U.; Perchellet, E.M.; Klish, D.S.; Johnson, J.M.; Perchellet, J.P. Carcinogenesis.1992, 13, 715.
CrossRef - Inoue, M.; Suzuki, R.; Koide, T.; Sakaguchi, N.; Ogihara, Y.; Yabu, Y. Biophys Res Commun.1994, 204, 898.
CrossRef - Seleem, H.S.; El-Inany, G.A.; El-Shetary, B.A.; Mousa, M.A. Chemistry Central Journal.2011, 5, 2.
CrossRef - Abdel-Wahab, B.F.; Awad, G.E.A.; Badria, F.A. Eur. J. Med. Chem.2011, 46, 1505.
CrossRef - Abu-Surrah, A.S.; Abu Safieh, K.A.; Ahmad, I.M.; Abdalla, M.Y.;Ayoub, M.T.; Qaroush, A.K.; Abu-Mahtheieh, A.M. Eur. J. Med. Chem.2010, 45, 471.
CrossRef - Ajani, O.O.; Obafemi, C.A.; Nwinyi, O.C.; Akinpelu, D.A. Bioorg. Med. Chem.2010, 18, 214.
CrossRef - Al-Said, M.S.; Bashandy, M.S.; Al-Qasoumi, S.I.; Ghorab, M.M. Eur. J. Med. Chem.2011, 46, 137.
CrossRef - Aslam, M.A.S.; Mahmood, S.U.; Shahid, M.; Saeed, A.; Iqbal, J. Eur. J. Med. Chem.2011, 46, 5473.
- CrossRef
- Cui, Z.; Li, Y.; Ling, Y.; Huang, J.; Cui, J.; Wang, R.; Yang, X. Eur. J. Med. Chem.2010, 45,5576.
CrossRef - Kaushik D.; Khan, S.A.; Chawla, G.; Kumar, S. Eur. J. Med. Chem.2010, 45, 3943.
CrossRef - Edrees, M.M.; Farghaly, T.A.; El-Hag, F.A.A.; Abdalla, M.M. Eur. J. Med. Chem.2010, 45, 5702.
CrossRef - Gregory Backes, L.; Donna Neumann, M.; BrankoJursic, S. Bioorg. Med.Chem. 2014, 22, 4629.
- Chavignon, O.; Teulade, J.C.; Madesclaire, M.; Geueiffier, A.; Blache, Y.; Viols, H.; Chapal, J.P.; Dauphin, G. J. Heterocycl. Chem.1992, 29, 691.
CrossRef - VeerabhadraSwamy, P.; Chandrasekhar, K.B.; Pullaiah, C.K. J. Applicable. Chem. 2015, 4, 492.
- Shrinivasan, D.; Sangeetha, N.; Suresh, T.; Lakshmanaperumalsamy, P. J. Ethnophrmacol.2001, 74, 217.
CrossRef - Litchfield, J.T.; Wilcoxon, E.J. J. Pharmacol. Exp. Ther. 1949, 96, 99.
- Raghavan, P.V. (2000) Expert Consultant, CPCSEA, OECD, guideline no 420.
- Jarret, R.J.; Keen, H.; Hardwick, C. Diabetes. 1970, 19, 724.
CrossRef - Mackener, C.R.; Saunders, R.N.; Haettinger, J.R..J. Toxi. Envi. Health. 1976, 2, 139.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









