Influence of pH Variation on Structural and Magnetic Properties of Ni-Zn Ferrite Nanoparticles Synthesized by Auto Combustion Method
Richa1, Anand K. Tyagi2 and Dharamvir Singh Ahlawat3
1Department of Physics, I.K. Gujral Punjab Technical University, Jalandhar, Punjab India.
2Department of Applied Sciences and Humanities, Shaheed Bhagat Singh State Technical Campus, Ferozepur-152002, Punjab, India.
3Department of Physics, Chaudhary Devi Lal University, Sirsa-125055, Haryana, India.
Corresponding Author E-mail: randeepkaur02@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/330135
Nickel zinc ferrite (Ni0.5Zn0.5Fe2O4) nanoparticles were synthesized via solution auto ignition combustion method. The ferrite samples were synthesized with different pH values 3, 5, 7 and 8. The as synthesized samples were calcined at 800oC for 4 hours. These synthesized nanopowder have been characterized by XRD, SEM and VSM and effect of pH value on the properties of the ferrite samples were studied. XRD pattern confirmed that samples were crystalline in nature. The crystallite size of ferrite powder increased with increased in basic nature of ferrite nanopaticles. Crystallite size of sample was found 17.4nm, 19.34nm, 20.50nm and 22.66nm at pH values 3, 5, 7 and 8 respectively. The magnetization of samples prepared at pH values 3, 5, 7 and 8 were found 55.490e.m.u./g, 61.420e.m.u./g, 65.541e.m.u./g and 66.512e.m.u./g respectively.
KEYWORDS:Solution-combustion method; Magnetic materials; X-ray diffraction Magnetic properties
Download this article as:| Copy the following to cite this article: Richa R, Tyagi A. K, Ahlawat D. S. Influence of pH Variation on Structural and Magnetic Properties of Ni-Zn Ferrite Nanoparticles Synthesized by Auto Combustion Method. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Richa R, Tyagi A. K, Ahlawat D. S. Influence of pH Variation on Structural and Magnetic Properties of Ni-Zn Ferrite Nanoparticles Synthesized by Auto Combustion Method. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=30546 |
Introduction
Soft ferrite materials draw more attention due to their unique properties [1]. Ferrite powder have attracted the researchers and investigators of different fields due to their numerous applications like memory system, medical science instrument etc. [2-7]. Ferrites are important materials due to their various practical applications, such as magnetic storage devices [8-9]. Parameters like composition, method of synthesis, substitution of different cations, annealing time and temperature, sintered density, porosity and crystallite size alter the properties of material [10-11]. Ferrites materials are comparatively economical for preparation of various types of sensors to probe, such as magnetic field [12], current [13], gas concentrations [14-15], heat treatment [16] and mechanical stresses [17], and. Ferrite temperature sensors used in biochemical applications [18-21]. Main reasons of being ferrite samples are attracting researchers due to it’s their high resistivity, applicability at higher frequency and higher corrosion resistance that makes them highly important for high frequency applications. Different method for synthesis of ferrite powder are co-precipitation [23], sol–gel method [22], micro emulsion method [26], hydrothermal [24, 25], reverse micelle [28], precursor [27], etc. Complex synthsization process and lower production rate are the main problems of these wet-chemical methods [29].Sol–gel auto-combustion or auto-ignition preparation method where the chemical sol–gel and ignition process is combined. This powder preparation shown great possibility in the synthesization of spinel type ferrite nanomaterials. This method of synthesis can be considered as solution combustion technique [30]. It has been applicable for the preparation of different spinel ferrite compounds NFe2O4, where N could be Zn, Ni, Co, Cu, Mg, Mn ion or its combination [31–42]. Attraction of researchers towards Ni-Zn ferrite among soft ferrites family, due to numerous important applications [43]. In the present course of work we have prepared the Ni-Zn ferrite (NZF) by solution auto combustion method. Normally, in ferrites nickel has a more tendency to occupy the octahedral sites whereas zinc occupies the tetrahedral site. Thus, nickel ferrite has an inverse spinel structure whereas zinc ferrite has a normal spinel structure. Hence, NZF have a mixed spinel ferrite structure. The tetrahedral sites are occupied by Zn2+ and Fe3+ whereas the octahedral sites are occupied by Ni2+ and Fe3+. The interactions between the ions in tetrahedral and octahedral sites can alter magnetic and electrical parameters of prepared nano-ferrites. The nickel zinc ferrite properties are also dependent on the methodology adopted for their synthesis, preparative conditions like pH of solution, sintering temperature and time, chemical composition, grain size. Novelty of work is that we have prepared the nickel zinc ferrite powder by economical method. Nickel zinc ferrite. The main objective is to study the effect of pH values on the structural and magnetic parameters of solution auto ignition derived NZF nanoparticles.
Experiemtnal Details
Materila and Methods
Nickel Nitrate Hexahydrate, Zinc Nitrate Hexahydrate, Iron Nitrate . Nonahydrate, Citric Acid(Himeida, AR), Ammonia Solution(Sigma Aldrich, 33%).
Preparation of NZF nanoparticles
Nickel zinc ferrite (Ni0.5Zn0.5Fe2O4) powder was prepared by solution auto-combustion technique. Metal nitrates Zn(NO3)2·6H2O, Ni(NO3)2·6H2O, Fe(NO3)3. 9H2O were used as starting materials. These nitrates were added separately in double distilled water and stirred for 20 minutes at 70oC [30]. Solution of citric acid in double distilled water poured into stirred metal nitrate solution, and then ammonia solution was added to continuous stirred solution for adjustment of pH value. The pH of solution was adjusted to 3, 5, 7 and 8 using ammonia. The mixed solution was kept onto a hot plate with continuous stirring at 100oC. After continuous approximate 7 hours of stirring the viscous brown black gel formed. After the formation of a highly dense sticky black brown gel, the temperature was raised to 120 °C. The temperature was then increased rapidly upto 200°C, huge amounts of gases like H2O, CO2 and N2 were produced and when heated at 250-300oC, the dried gel burnt out completely to form a loose powder. The loose powder was ground in pestle motor for 30 minutes to form fine powder. Finally, the as prepared fine powder calcined at 800°C for 4 h (heating rate of 10°C/min).
Characterization Techniques
XRD
The structural parameters of synthesized NZF were investigated by X-ray diffraction (XRD; The Bruker D8 X-ray diffractometers a). The XRD pattern of samples were taken in the angle range 10-80° with 2°/min.
SEM
The morphological nautre of samples was scanned using scanning electron microscopy equipped with an energy dispersive X-ray spectrometer (SEM, JEOL).
VSM
Magnetic nature of prepared nanoparticles were studied by a vibrating sampler magnetometer (VSM PAR-155 at IIC, IIT, Roorkee) Range: 0.00001 to 10000 e.m.u., Magnetic field: 10 to +10 kOe , Temperature range : Room Temperature).
Results and Discussions
XRD
Fig.1 demonstrates the XRD pattern of NZF samples synthesized at different pH values (3, 5, 7 and 8). All the prepared samples NZ1(pH3), NZ2(pH5), NZ3(pH7) and NZ4(pH8) were crystalline in nature. The peaks of the XRD were corresponding to (111), (220), (311), (222), (400), (422), (511) and (440) planes. The highest intense peak corresponding to (311) plane. The width of the peaks decrease with increase in pH value. The width of diffraction maxima peaks (311) become reduced and intensity of peaks increased as the pH increases. The average crystallite size of NZF and crystallinity are increased with increase in pH value of samples. The crystallite size (D) was measured by using Debye Scherer formula [44]
![]()
Where k is the structure factor, β is the full width at half maxima, λ is the X-Ray wavelength, θ is the Brag Diffraction angle. Due to the fast combustion rate and high ignition temperature with increasing pH, higher pH produced the powder with larger crystallite size and good crystallinity. Crystallite size of sample NZ1, NZ2, NZ3 and NZ4 were found 17.4nm, 19.34nm, 20.50nm and 22.66nm respectively. Similar type of results was also reported by Nilar Lwin et al. [45]. Fig. 2 shows the shifting of peak (311) with various pH values. Table 1 demonstrates the structural parameters like interplanar spacing, crystallite size of samples NZ1, NZ2, NZ3 and NZ4. Various structural parameters like crystallite size, lattice constant demonstrates in table 1.
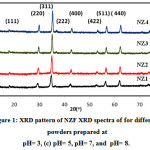 |
Figure 1: XRD pattern of NZF XRD spectra of for different powders prepared at pH= 3, (c) pH= 5, pH= 7, and pH= 8.
|
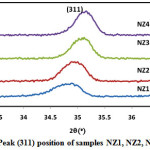 |
Figure 2: Peak (311) position of samples NZ1, NZ2, NZ3 and NZ4. |
Table 1: Structural parameters of samples NZ1, NZ2, NZ3 and NZ4.
|
Sample |
Peak position |
FWHM (o) |
Crystallite size |
Strain |
Interplanar Spacing d (Ao) |
Lattice Constant a(Ao)
|
|
NZ1(pH3) |
34.79 |
0.49 |
17.4 |
0.0070 |
2.5750 |
8.5440 |
|
NZ2(pH5) |
34.96 |
0.45 |
19.34 |
0.0062 |
2.5641 |
8.5041 |
|
NZ3(pH7) |
35.03 |
0.42 |
20.50 |
0.0066 |
2.5622 |
8.4977 |
|
NZ4(pH8) |
35.10 |
0.38 |
22.66 |
0.0060 |
2.5512 |
8.4613 |
SEM
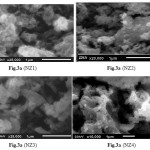 |
Figure 3 |
The SEM images of NZF heat-treated at 800°C are presented in Fig.3a, 3b, 3c and 3d. The SEM demonstrates that the NZF particles have a strong tendency to hold together, forming agglomerates because of the magnetic interactions between ferrite particles during preparation process [46-47]. Grain size of NZ1, NZ2, NZ3 and NZ4 were found 0.09µm, 0.1µm, 0.13µm and 0.14µm respectively. These voids are also present in the as synthesized sample. This may be attributed to the interaction between the magnetic nanoparticles, in turn these particles grow to some extent to form larger particles at high pH of the solution [48].
Magnetic properties
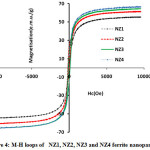 |
Figure 4: M-H loops of NZ1, NZ2, NZ3 and NZ4 ferrite nanoparticles. |
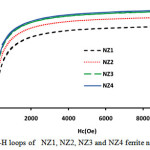 |
Figure 5: M-H loops of NZ1, NZ2, NZ3 and NZ4 ferrite nanoparticles. Click here to View figure |
The magnetic parameters of ferrites nanoparticles were altered by different parameters like crystallite size, density, super exchange interactions between A and B-sites and composition. Magnetic hysteresis loops of NZF samples calcined at 800°C were obtained at room temperature by vibrating sample magnetometer (VSM) technique and are demonstrates in Fig.5 and Fig.6. The magnetic parameters like saturation magnetization, coercivity were measured from M-H loops listed in Table 2.
Table 2: Structural parameters of samples NZ1, NZ2, NZ3 and NZ4.
|
Sample |
Saturation Magnetization (e.m.u./g) |
Coercivity (Oe) |
|
NZ1(pH3) |
55.490 |
30.50 |
|
NZ2(pH5) |
61.420 |
32.50 |
|
NZ3(pH7) |
65.541 |
35.23 |
|
NZ4(pH8) |
66.512 |
33.40 |
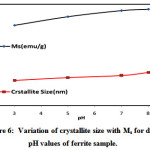 |
Figure 6: Variation of crystallite size with Ms for different pH values of ferrite sample. |
Magnitude of magnetization of Ni-Zn ferrite nanoparticles depend on occurrence of Fe ions at A or B site. More the migration of Fe3+ ions from A to B site results in enhancement in magnetization, due to more ions at B site as compared to A site. Hence total magnetization i.e. Ms=MB −MA increased. Nilar et. al have also reported the similar type of results[44, 49, 50]. Fig.7 demonstrates the graph between magnetizationand crystallite size with different pH values 3, 5, 7 and 8.
Conclusions
The NZF samples were successfully prepared with different pH values by solution auto ignition method. The impact of pH on the magnetic and structural parameters of NZF was investigated. The XRD results clearly shows that the crystallinity and average crystallite size of powders increases with an increase in pH value. The crystallite size of ferrite powder increased with increased in basic nature of NZF. Crystallite size of sample was found 17.4nm, 19.34nm, 20.50nm and 22.66nm at pH values 3, 5, 7 and 8 respectively. All the prepared samples NZ1, NZ2, NZ3 nad NZ4 are single-phase ferrites with cubic spinel structures. Auto ignition or auto combustion process resulted in the formation of nano-sized (17.34–22.66 nm), highly reactive and crystalline nickel zinc ferrite. The magnetization of samples NZ1, NZ2, NZ3 and NZ4 were measured 55.490e.m.u./g, 61.420e.m.u./g, 65.541e.m.u./g and 66.512e.m.u./g respectively.
Acknowledgements
This research work has been supported by the infrastructure of I.K. Gujral, Punjab Technical University, Jalandhar (Kapurthala). We are also very grateful to TEQIP, MHRD/World Bank Project for providing the necessary research facilities.
References
- Yeon, I; Kim, KD; Lee, CS.; Physica B: Physics of Condensed Matter. 2003, 337, 42-51.
CrossRef - Li, S; Liu, L; John, VT; O’Connor, CJ; Harris, VG.; IEEE Transactions on Magnetics (New York). 2001, 37, 2350-2352.
CrossRef - Didukh, P.; Greneche, JM.; Slawska-Waniewska, A; Fannin, PC; Casas, LI.; J. of Mag. and Magnetic Mat. 2002, 242, 613-616.
CrossRef - Neveu, S.; Bee, A.; Robineau, M.; Talbot, D.; J. of Colloid and Interface Sci. 2002, 255, 293-298.
CrossRef - Rahmayeni; Zulhadjri; Jamarun, N..; Emriadi; Arief, S.; Oriental J. Of Chem. 2016, 32, 1411-1419.
CrossRef - Pathmamanoharan, C.; Philipse, A.P.; J. of Colloid and Interface Science. 1998, 205, 340-353.
CrossRef - Arora A. K.; Devi S.; Jaswal V. S.; Singh J.; Kinger M.; Gupta V. D. Oriental Journal of Chemistry. 2014, 30, (4), 1671-1679
- Kumar, A.; Shrotri, P.S.; Deshpande, CE; Date, SK.; J. of Applied Phys. 1997, 81, 4788–4790 .
CrossRef - Gubbala, S.; Nathani, H.; Koizol, K; Misra, R.D.K.; J. Phys. B. 2004, 348,317–328.
CrossRef - Parvatheeswara Rao, B.; Rao, P. S. V.; Rao, K. H.; J. Phys. 1997, IV France7, CI-241.
- Goldman, A.; Handbook of Modern Ferromagnetic Materials, Springer Science + Business Media, New York, 1999.
- Sedlar, M.; Matejec, V.; Paulicka, I.; Sens. Act. A, 2000, 84, 297–302.
CrossRef - Brito, V. L. O.; Migliano, A. C. C.; Lemos, L. V.; Melo, F. C. L.; Progress In Electromagnetics Research, 2009, 91, 303–318.
CrossRef - Zhang, G.; Li, C.; Cheng, F.; Chen, J.; Sens. Act. B, 2007, 120, 403–410.
CrossRef - Arshak, K.; Gaidan, I.; Mat. Sci. Eng. B 2005, 118, 44–49 .
CrossRef - Shin, H.-S.; U.S. Patent No.5, 1998, 775, 810.
- Bien ́kowski, A.; Szewczyc, R.; Sens. Act. A, 2004, 113, 270–276.
- Kim, Y. H.; Hashi, S.; Ishiyama, K.; Arai, K. I. ; Inoue, M.; IEEE Trans. Magn. 2000, 36, 3643–3645.
CrossRef - Osada, H.; Seki, K.; Matsuki, H.; Kikuchi, S.; Murakami, K.; IEEE Trans. Magn. 1995, 31, 3164–3166.
CrossRef - Osada, H.; Chiba, S.; Oka, H.; Hatafuku, H.; Tayama, N.; Seki, K; J. Magn. Magn. Mater. 2004, 272, 1761–1762.
CrossRef - Van Uitert, L. G. ; J. Chem. Phys. 1956, 24, 306.
CrossRef - Gul, I. H.; Ahmed, W.; Maqsood, A.; J. of Mag. and Magnetic Mat. 2008, 320, 270–275
CrossRef - Zahi, S.; Hashim, M; Daud, A. R.; J. of Mag. and Magnetic Mat. 2007, 308, 177–182
CrossRef - Kosak, A.; Makovec, D.; Znidarsic, A.; J. of the European Ceramic Soc. 2004, 24, 959–962
CrossRef - Jiao, X; Chen, D.; Hu, Y.; Mate. Research Bulletin, 2002, 37, 1583–1588
CrossRef - Takayama, A.; Okuya, M; Kaneko, S.; Solid State Ionics, 2004, 172, 257–260
CrossRef - Thakur, S; Katyal, S .C.; Singh, M.; J. of Mag. and Magnetic Mat. 2009, 321, 1–7.
CrossRef - Sarangi, P .P.; Vadera, S. R.; Patra, M K; Powder Technology 2010, 203, 348–353
CrossRef - Balaji, S; Kalai Selvan, K; John Berchmans, L.; Materials Science and Engineering B, 2005, 119, 119–124.
CrossRef - Aruna, S T; Mukasyan, .A. S.; Current Opinion in Solid State and Mat. Sci. 2008, 12, 44–50.
CrossRef - Randhawa, B S; Dosanjh, H.S.; Kumar, N.; J. of Radio analytical and Nuclear Chem. 2007, 274, 581–591
CrossRef - Lee, S.P; Chen, Y.J; Ho, C.M.; Materials Science and Engineering B 2007, 143, 1–6.
CrossRef - Sutka, A.; Mezinskis, G.; Pludons, A.; Power Engi. 2010, 56, 254–259.
- Sutka, A.; Gross, K. A.; Mezinskis, G.; Physica Scripta, 2011, 83, 025601.
CrossRef - Thant, A. A.; Srimala, S.; Kaung, P.; J. of the Australian Ceramic Soc. 2010, 46, 11–14
- Nayak, P. K.; Materials Chemistry and Physics 2008, 112, 24–26.
CrossRef - Shobana, M.K.; Rajendran, V; Jeyasubramanian, K.; Mat. Letters 2007, 61, 2616–2619.
CrossRef - Mallapur, M. M; Shaikh, P.A.; Kambale, R C; J. of Alloys and Compounds 2009, 479, 797–802.
CrossRef - Yue, Z.; Zhou, J.; Li, L.; Mat. Sci. and Eng. B 2001, 86, 64–69.
CrossRef - Azadmanjiri, J; Salehani, H. K.; Barati, M R; Mat. Letters 2007, 61, 84–87
CrossRef - Yue, Z, Zhou J, Li, L.; Zhang, H; Gui, Z; J. of Mag. and Magnetic Mat. 2000, 208, 55–60.
CrossRef - Selvan, R. K.; Augustin, C. O.; Berchmans, L. J.; Saraswathi, R.; Mat. Research Bulletin 2003, 38, 41–54.
CrossRef - Yadoji, P.; Peelamedu, R.; Agrawal, D.; Roy, R.; Mater. Sci.Eng. B 2003, 98, 269.
CrossRef - Lwin, N; Othman, R.; Noor, A.F.M.; Sreekantan, S.; Yong, T.C.; Singh, R; Tin, C.C.; , Mat. Characterization 2015, 110 , 109–115
CrossRef - Bendonia, R.; Barthélémyc, A.; Sangiorgia, N.; Sangiorgia, A.; Sansona, A. J.of Photochem. and Photobio. A: Chem. 2016, 330, 8–14.
- Hsiang, H.I. ; Tsai, J.Y; J. Mater. Sci. 2006, 41, 6339–6346.
CrossRef - Radyum, I.; Putri, R.A.S. ; Wahyu, B.W.; Agus, S. ; Nurul, T.R.; Int. J. of Eng. & Tech. 2012 12, 5–9.
- Gharagozlou, M.; Bayati, R; Superlattices Microstruct., 2015, 78, 190.
CrossRef - Vijaya,K.; Paramesh, D.; Reddy, PV; World J. Nano Sci. Eng.2015 7, 68–77.
- Jia, B.; Gao, L.; Crystal Growth Design 2008, 8, 1372–1376.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









