Different Applications of Magnetic Nanoparticles in the Rapid Monitoring of Ochratoxin A
Azam Sargazi and Mostafa Heidari Majd
Department of Medicinal Chemistry, Faculty of Pharmacy, Zabol University of Medical Sciences, Zabol, Iran.
Corresponding Author E-mail: mostafamajd@live.com
DOI : http://dx.doi.org/10.13005/ojc/330141
Article Received on :
Article Accepted on :
Article Published : 16 Feb 2017
Toxic effects of mycotoxins are still an unresolved subject for health of humans and animals. Therefore, several methods has been created to determine and eliminate mycotoxins from foodstuffs, and also are developing. For instance, utilization of magnetic nanoparticles (MNPs) when are combined with different analytical tools is one of the developing methods which could be useful for extraction of mycotoxins. As in some methods, they were employed as newfound adsorbents in magnetic solid phase extraction approaches.In some other methods, MNPs were used for immobilization of antibodies to enhance the efficiency of the analytical tools such as square wave voltammetry. Or in another method, amine-functionalized MNPs were reacted with ochratoxin A (OTA) to enhance performance of chemiluminescence immunoassay. Results of all methods were showed that utilizing of MNPs could reduce cost, easy preparation, and increase the stability, speed, sensitivity, precision and reproducibility. So, these new methods have elicited great hopes to be suitable alternative for older methods.
KEYWORDS:Ochratoxin A; Extraction; MagneticSolid Phase Extraction; Aptamer-Targeted Magnetic Nanospheres; Chemiluminescence Immunoassay
Download this article as:| Copy the following to cite this article: Sargazi A, Majd M. H. Different Applications of Magnetic Nanoparticles in the Rapid Monitoring of Ochratoxin A. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Sargazi A, Majd M. H. Different Applications of Magnetic Nanoparticles in the Rapid Monitoring of Ochratoxin A. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=30240 |
Introduction
Molds are filamentous fungi which found in many feedstuffs, and produce toxins called mycotoxins. However, mycotoxins are classified as secondary metabolites and their function are not related to the mold’s existence, but they can affect the health of animals when they consume from these contaminated feeds 1. Mold growth can occur during storage and also during feed processing under warm and humid conditions. Therefore, mycotoxin production could be occur in un-cleaned silos, shipment systems and also food factory; so it is possible that more than one mycotoxin exist in every infected feeds 2,3.
On the other hand, many mycotoxins are known which their toxin production and also their structural chemistry depend on the susceptibility, metabolism and defense mechanisms 4. So, they remain in the food chain, even in cooking and freezing treatments. Mycotoxins also resist dissociation in digestive tract which can have a noticeable effect on immune system of animals and humans 5,6.
Several mycotoxins which can contaminate foods are included ochratoxins, aflatoxins, zearalenone and deoxynivalenol 7. Meanwhile, Ochratoxins are derivative of isocoumarin which produced by several fungi belonging to Aspergillus or Penicillium species8. Ochratoxins, based on chemical structures, can be divided into three secondary metabolite forms, including A, B, and C 2;but Ochratoxin A (OTA) is a prevalent and toxic component of this family which can affect mainly the kidney9. OTA have been discovered mainly in grape juice, coffee, cereal, milk, beer, pulses, spices, dried fruits and also meat products 10,11. On the other hand, OTA can be the reason of serious diseases in humans such as hepatotoxic, nephrotoxic, immunotoxic, etc, because of its strong affinity to serum albumin, stability against acidic degradation and also stability during food processing operations 12.Therefore, it is important that its amount be measured by an analysis method with well detection limit.
Comparison of Analytical Methods used for Detection of Ochratoxin A
Various methods have been reported for laboratory assessment of ochratoxin A in food samples, including high-performance liquid chromatography (HPLC), capillary electrophoresis (CE), gas chromatography-mass spectrometry (GC-MS), thin layer chromatography (TLC), enzyme-linked immunosorbent assay (ELISA), fluorimetry (FL), immunoassay based on fluorescence polarization (FPIA), and immunosensor based on surface plasmon resonance (SPR) 13.One of the most common approaches among these mentioned methods for detection of OTA is the HPLC with fluorescence detector (HPLC/FLD) in which OTA must be extracted by the immunoaffinity column (IAC) 14.Briefly in this method, the sample solutions are passed through the columns filled with immobilized antibody against the ochratoxin A. Sample matrix is washed out by water or an aqueous buffer and OTA is gathered by methanol 15. Though this method is sensitive and selective, but has some disadvantagessuch as time-consuming, high cost, and contamination of IAC with the ethyl ester residue of OTAwhich extensively reduce its efficiency 14.
Over the last decade, some alternative methods have been created for extraction and detection of ochratoxin A, but none have been so successful.For instance, TLC and CE methods aren’t very sensitive, the FL method has higher sensitivity than that of the TCL, but its selectivity is low, 16 and in ELISA method, due to the poor chemical/physical stability of the antibodies or enzymes, their use in acidic or basic conditions and also organic solvents are limited17. Therefore, the investigation of the modern methods, such as magnetic solid phase extraction (MSPE) which has got many benefits such as low toxicity, simple preparation and low price, is necessary for detection of OTA14.
Magnetic nanoparticles have some advantages such as suitable nano-scale size, high hydrophilicity and more surface modification with functionalized groups, which make them a good candidate for use in extraction18,19.Also, other abilities like large surface area to the volume ratio, rapid extraction and high extraction efficiencies, could be helpfulfor this purpose.So, in this paper, we review all the methods in which MNPs are used for extraction and measurement of OTA in foods and biological fluids.
Detection of Ochratoxin Aby MagneticSolid Phase Extraction
A novel, fast, simple, and environmentally friendly method based on modified Fe3O4 nanoparticles was introduced for extraction of OTA by Mashhadizadeh et al.at 2013 20. In this method, a HPLC-fluorescence detection (Waters 474) system with RP-18e column was employed for determination of OTA after extraction by MNPs.For this purpose, Fe3O4 nanoparticles were synthesized and then were modified with 3-(trimethoxysilyl)-1-propanethiol (MSPT) and ethylene glycol bis-mercaptoacetate (EGBMA), respectively.Figure 1 represents the SEM micrographof EGBMA-MSPT-MNPs sorbents with average size ~ 40 nm.
 |
Figure 1: SEM micrograph of EGBMA-MSPT-MNPs (WD19) sorbents Click here to View figure |
Usual step for the extraction of OTA, before employing the HPLC, is immunoaffinity column (IAC) clean-up procedure which has some disadvantages 21.But, in this work, MNPs were selected instead IACs to provide a successful interaction with OTA. The OTA as a polar molecule contains both ionizable and hydrophobic sections, therefore authors hypothesize that magnetic adsorbents (EGBMA-MSPT-MNPs) with both electrostatic and hydrophobic properties could be useful for interaction with OTA.In a similar work which was done by Sargazi et al, dopamine loaded MNPs were synthesized to be utilized as a nanosized functional adsorbent in extraction of OTA22. Because in proper condition, the terminal amino groupof dopamine has the positively charged (-NH3+) 18,23 and can interact with OTA which exists in anionic form (OTA–)24.
After examination of different parameters which affect the efficiency of new adsorbents, the selectivity of magnetic sorbents for OTA in real samples such as cereal and rice were confirmed. Low detection limit (LOD < 0.07 ng mL−1), high precision (RSD < 7%) and good trueness (recoveries > 87%) of tests showed that magnetic solid phase extraction, as an alternative cleanup method in comparison to immunoaffinity column procedure, possess the ability to extract OTA from real samples.
Magnetic Solid Phase Extraction by Aptamer-Targeted Magnetic Nanospheres
In this work the aptamers for OTA were immobilized onto the carboxyl group-modified magnetic nanospheres and were utilized for magnetic solid-phase extraction (MSPE) procedure. Figure 2 depicts the preparation of the magnetic nanospheres–aptamer (MNS-aptamer) adsorbents25.
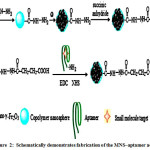 |
Figure 2: Schematically demonstrates fabrication of the MNS–aptamer adsorbents |
Clean up by the MSPE can save the time of extraction, because MNPs can be readily collected by an external magnetic field, and also further purification such as centrifugation or filtration isn’t necessary for this procedure26. Aptamers that bind to OTA, in the presence of Mg2+, show a G-quarter structure, and therefore, occur the special OTA–aptamer interaction with high efficiency. Finally, the magnetic nanospheres (MNS) are collected using an external magnetic field, and quantified by HPLC.
The specificity of the magnetic aptamer toward OTA was examined in the presence of several compounds which have structural similarities to OTA.This test hinted that presence of other mycotoxins like zearalenone (ZEN) and deoxynivalenol (DON) have no impact on performance of MNS–aptamer toward ochratoxin A.
The MNS–aptamer sorbent, because of both short diffusion path and large surface area to volume ratio of MNPs 27, shows high extraction capacity (500 ng OTA/ml);and it seems that development of method for detection of OTA in real food samples, be effective.Therefore, the ability of this method for real samples such as cereal and coffee, also was examined.The recovery amounts of OTA in three matrix samples were ranged from 67.2% to 90.4%and showed the acceptable affinity of sorbent to the target molecule.
All these results are confirmed that magnetic solid phase extraction by MNS–aptamer has the combined advantages of easy and inexpensive preparing in comparison to immunoaffinity columns.
Ochratoxin A Determination by an Electrochemical Method Using Modified Magnetic Nanoparticles
In this procedure for determination ofOTA in food samples, an electrochemical technique was utilized where modified magnetic nanoparticles (MNPs) were combined with square wave voltammetry (SWV)28.Although, square wave voltammetry (SWV) hasn’t proper detection limitsfor OTA determination, but physical properties of MNPs can enhance the analytical applications of SWV as a beneficial tool for detection of ochratoxinA 28,29.Briefly, the surface of MNPs was modified by mouse monoclonal anti-OTA antibody to improve the performance of the SWV.For the pre-concentration and transfer into electrochemical cells, the OTA was adsorbed to the anti-OTA antibody and then was eluted by desorption solvent. Finally, existence of OTA was monitored by measuring the oxidation rate of it in acidic conditions via standard glassy carbon (GC) electrode (Fig. 3).
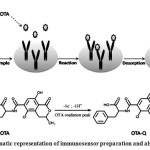 |
Figure 3: Schematic representation of immunosensor preparation and also oxidation of OTA Click here to View figure |
Due to the phenolic group of isocoumarin moiety and the carboxyl group of phenylalanine moiety in the OTA structure, it has weak acidic properties; so, an acidic solution composed of perchloric acid was used for desorption of OTA from MNPs. Because, according to pH-partition theory, acids are un-ionized in acidic solutions and so the lowest amount of absorption occurs in the pH range from 1.0 to 5.0 22. Since, this new method is inexpensive, reproducible and accurate, could be a good candidate for the determination of OTA in real samples in comparison with older methods.
Use of Magnetic Nanoparticles in Detection of Ochratoxin A by a Label-Free Immunosensor
Nearly the same of before procedure, magnetic properties of MNPs are used for immobilization of anti-OTA mouse monoclonal antibody on external layer of gold electrode30.In order to reduce the direct binding of ochratoxin A to Au working electrodeand prevention of direct oxidation at the naked electrode, the surface of electrode was covered utilizing bovine serum albumin (BSA)before attaching to MNPs 31,32. Figure 4 represents all steps of the preparation of the immunosensor.
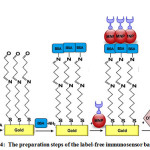 |
Figure 4: The preparation steps of the label-free immunosensor based on MNPs Click here to View figure |
As a great advantage,The surface of biosensors can be renewed rapidly by an external magnet, when MNPs respond to the applied magnetic field33.Also, MNPs can provide several functional groups on the surface of electrode which lead to increasing the stability and number of the surface-bound antibodies 34.On the other hand, electrochemical detection of OTA at pH ≥ 7 was simply possible, because molecule at pH above 7 shows both mono-anionic (OTA−) and di-anionic (OTA2−) forms. Therefore, on the surface of electrode, all existing active sites related to the antibodies were occupied, when concentration was higher than 5 ng/mL of OTA.
Cyclic voltammetry (CV) analysis represents that the bio-magnetic shell has promising reproducibility which is confirmed by standard deviation of 6.5% (n = 5). According to quick and valid results plus sensitivity, accuracy and reproducibility of this magnetic immunosensor, authors have suggested it as an applicable bio-analytical tool for detection of OTA in real samples.
Detection of Ochratoxin A by Combining Magnetic Nanoparticles and Rolling Circular Amplification
ELISA (enzyme-linked immunosorbent assay) traditionally utilize the antibodies, but its storage is hard, and its preparation needs to long time and high cost 35.As a new technique with excellent sensitivity for detection of OTA, Yao et al combined the magnetic purification with the system of rolling circular amplification (RCA) to reduce the noise and to enhance the signal. Since the magnetic carriers have got functional groups like ion exchanger ligands, hydrophobic groups or bio-polymericagents, the purification can be done in one step without utilizing centrifuges, filters or other equipment 36.For this purpose, carboxyl–MNPs were prepared by solve-thermal protocol 37and were surface modified via the RCA primercontaining the specific aptamer related to OTA 38.
Sensitive detection of OTA was carried out using the above RCA system and alsoaQDs labeled ssDNA probe with fluorescent propertiesto improve sensitivity of system and to amplify signals.Figure 5 shows that by using of QDs, fluorescence intensity related to RCA system is decreased, when the concentration of OTA is increased. Of note, by purifying the residual QDs labeled probes through the magnetic separation operation, the interference of background fluorescent noise was reduced and thus efficiency and sensitivity for OTA detection were improved.
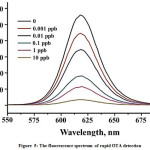 |
Figure 5: The fluorescence spectrum of rapid OTA detection |
Theoretically, in this ultrasensitive detection, ochratoxin A combine with the primer and prevent from fluorescent emission, but in the absence of OTA, the primer onto magnetic nanoparticles would react with the RCA padlock which lead to strong fluorescent emission. So in general, this integrated platform could be useful for rapid detection of OTA in about 80 min, and could be a viable alternative for traditional methods of OTA detection.
Determination of Ochratoxin A by Chemiluminescence Immunoassay Technique Using Magnetic Nanoparticles
In this technique, a chemiluminescence (CL) immunoassay was coupled with amine-functionalized magnetic nanoparticles (MNPs) for chemically extraction and determination of OTA 39. Generally for the extraction of OTA by MNPs, the carboxylic acid of OTA was activated by EDC (N-(3-dimethyl amino propyl)-N-ethyl carbodiimide hydrochloride) and NHS (N-hydroxysuccinimide) (Fig. 6, step 1) for directly reaction of the carboxylic group of OTA with amine-functionalized MNPs. Thereupon, for CL detection, monoclonal primary antibody and horseradish peroxidase (HRP) conjugated polyclonal secondary antibody were respectively reacted with the OTA-MNPs (Figure 6, step 2 and 3). When, this procedure was utilized for rice samples, the average limit of detection of 1.39 pg mL-1 (R2=0.988) was obtained which confirmed this developed procedure as a useful tool for the examination of various toxins like OTA.
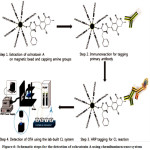 |
Figure 6: Schematic steps for the detection of ochratoxin A using chemiluminescence system |
Conclusion
Detection of OTA in food samples is very critical because it can act as a potential carcinogen and neurotoxic. Up to now, scientists have done a lot of effort to assay OTA by different methods, but most of them have many disadvantages such as lack of selectivity and sensitivity or require to expensive apparatus and complex procedures. Recently, novel methods, as nanotechnologies, are combined with usual detection methods to advance them to higher levels. Our review confirmed that utilize of MNPs in some procedures such as aptamer-based immunosensor, chemiluminescence immunoassay, square wave voltammetry or even solid phase extraction can reduce the cost and facilitate the preparation. On the other hand, it could increase the stability of the antibodies on surface of sensors and also increase the speed, sensitivity, precision and reproducibility of these approaches.
References
- Binder, E. M. Animal feed science and technology. 2007, 133, 149-166
CrossRef - Duarte, S.; Pena, A.; Lino, C. Food Microbiology. 2010, 27, 187-198
CrossRef - Richard, J. L. International journal of food microbiology. 2007, 119, 3-10
CrossRef - Hussein, H. S.; Brasel, J. M. Toxicology. 2001, 167, 101-134
CrossRef - Bullerman, L. B.; Bianchini, A. International Journal of Food Microbiology. 2007, 119, 140-146
CrossRef - Ghali, R.; Hmaissia-Khlifa, K.; Ghorbel, H.; Maaroufi, K.; Hedili, A. Food Control. 2008, 19, 921-924
CrossRef - Gambacorta, S.; Solfrizzo, H.; Visconti, A.; Powers, S.; Cossalter, A.; Pinton, P.; Oswald, I. World Mycotoxin Journal. 2013, 6, 299-308
CrossRef - Li, S.; Marquardt, R.; Frohlich, A.; Vitti, T.; Crow, G. Toxicology and applied pharmacology. 1997, 145, 82-90
CrossRef - Heussner, A. H.; Bingle, L. E. Toxins. 2015, 7, 4253-4282
CrossRef - Ringot, D.; Chango, A.; Schneider, Y.-J.; Larondelle, Y. Chemico-biological interactions. 2006, 159, 18-46
CrossRef - Gilbert, J.; Brereton, P.; MacDonald, S. Food Additives & Contaminants. 2001, 18, 1088-1093
CrossRef - Il’ichev, Y. V.; Perry, J. L.; Rüker, F.; Dockal, M.; Simon, J. D. Chemico-biological interactions. 2002, 141, 275-293
CrossRef - Wang, C.; Qian, J.; Wang, K.; Wang, K.; Liu, Q.; Dong, X.; Wang, C.; Huang, X. Biosensors and Bioelectronics. 2015, 68, 783-790
CrossRef - González-Peñas, E.; Leache, C.; Viscarret, M.; De Obanos, A. P.; Araguás, C.; De Cerain, A. L. Journal of Chromatography A. 2004, 1025, 163-168
CrossRef - Bazin, I.; Nabais, E.; Lopez-Ferber, M. Toxins. 2010, 2, 2230-2241
CrossRef - Wang, Q.; Chen, M.; Zhang, H.; Wen, W.; Zhang, X.; Wang, S. Analytical Methods. 2015, 7, 10224-10228
CrossRef - Xie, C.; Li, H.; Li, S.; Gao, S. Microchimica Acta. 2011, 174, 311-320
CrossRef - Sargazi, A.; Kuhestani, K.; Nosrat Nahoki, T.; Heidari Majd, M. International Journal of Pharmaceutical Sciences and Research. 2015, 6, 5047-5055
- Heidari Majd, M.; Akbarzadeh, A.; Sargazi, A. Artificial Cells, Nanomedicine and Biotechnology. 2017, 45, 441-447
CrossRef - Mashhadizadeh, M. H.; Amoli-Diva, M.; Pourghazi, K. Journal of Chromatography A. 2013, 1320, 17-26
CrossRef - Hackbart, H.; Prietto, L.; Primel, E. G.; Garda-Buffon, J.; Badiale-Furlong, E. Journal of the Brazilian Chemical Society. 2012, 23, 103-109
CrossRef - Sargazi, A.; Aliabadi, A.; Rahdari, A.; Allahdini-Hesaroiyeh, S.; Nejati-Yazdinejad, M.; Heidari Majd, M. Journal of the Brazilian Chemical Society. 2016, 110
- Saei, A. A.; Barzegari, A.; Majd, M. H.; Asgari, D.; Omidi, Y. Journal of Nanoparticle Research. 2014, 16,
- Poor, M.; Kunsagi-Mate, S.; Szente, L.; Matisz, G.; Secenji, G.; Czibulya, Z.; Koszegi, T. Food Chem. 2015, 172, 143-149
- Wu, X.; Hu, J.; Zhu, B.; Lu, L.; Huang, X.; Pang, D. Journal of Chromatography A. 2011, 1218, 7341-7346
CrossRef - Han, Q.; Wang, Z.; Xia, J.; Chen, S.; Zhang, X.; Ding, M. Talanta. 2012, 101, 388-395
CrossRef - Jiao, Z.; Jiao, S.; Guo, Z.; Chen, H.; Zhang, N.; Huang, W. Food Analytical Methods. 2016, 1-7
- Fernández-Baldo, M. A.; Bertolino, F. A.; Messina, G. A.; Sanz, M. I.; Raba, J. Talanta. 2010, 83, 651-657
CrossRef - Anjo, D. M.; Kahr, M.; Khodabakhsh, M.; Nowinski, S.; Wanger, M. Analytical chemistry. 1989, 61, 2603-2608
CrossRef - Zamfir, L.-G.; Geana, I.; Bourigua, S.; Rotariu, L.; Bala, C.; Errachid, A.; Jaffrezic-Renault, N. Sensors and Actuators B: Chemical. 2011, 159, 178-184
CrossRef - Perrotta, P. R.; Vettorazzi, N. R.; Arévalo, F. J.; Granero, A. M.; Chulze, S. N.; Zón, M. A.; Fernández, H. Electroanalysis. 2011, 23, 1585-1592
CrossRef - Ramírez, E. A.; Zón, M. A.; Ulloa, P. A. J.; Squella, J. A.; Vergara, L. N.; Fernández, H. Electrochimica Acta. 2010, 55, 771-778
CrossRef - Santandreu, M.; Solé, S.; Fàbregas, E.; Alegret, S. Biosensors and Bioelectronics. 1998, 13, 7-17
CrossRef - Yu, D.; Blankert, B.; Bodoki, E.; Bollo, S.; Viré, J.-C.; Sandulescu, R.; Nomura, A.; Kauffmann, J.-M. Sensors and Actuators B: Chemical. 2006, 113, 749 754
CrossRef - Zhao, Q.; Lv, Q.; Wang, H. Analytical chemistry. 2013, 86, 1238-1245
CrossRef - Safarik, I.; Safarikova, M. Biomagnetic Research and Technology. 2004, 2, 7
CrossRef - Liu, J.; Sun, Z.; Deng, Y.; Zou, Y.; Li, C.; Guo, X.; Xiong, L.; Gao, Y.; Li, F.; Zhao, D. Angewandte Chemie. 2009, 121, 5989-5993
CrossRef - Yao, L.; Chen, Y.; Teng, J.; Zheng, W.; Wu, J.; Adeloju, S. B.; Pan, D.; Chen, W. Biosensors and Bioelectronics. 2015, 74, 534-538
CrossRef - Kim, S.; Lim, H. Talanta. 2015, 140, 183-188
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









