Construction and Performance Characterization of Ion Selective Electrodes for Potentiometric Determination of Citapolarm Hydrobromide in Biological Fluids
Amani S . Alturiqi1,2, Samar O. Aljazzar1,2, Reda A. Ammar1,2 and Nuwair khalaf3
.1Department of Chemistry, College of Science, Princess Nourah Bint Abdul Rahman University, Riyadh, Saudi Arabia
2Deanship of Scientific Research, Princess Nourah Bint Abdul Rahman University, Riyadh, Saudi Arabia.
.3Chemistry Department, College of Science, King Saud University, Riyadh, Saudi Arabia
Corresponding Author E-mail: raammar@pnu.edu.sa
DOI : http://dx.doi.org/10.13005/ojc/330138
A coated-wire ion selective electrode based on ion-pair complex of citapolarmhydrobromide (CT)with sodium tetraphenyl borate as electroactive material in the presence of dioctylphthalate (DOP) as the plasticizing solvent mediatorwas prepared. The electrode displays Nernstian response of 56.83 mV/decade over the concentration range of 1.0×10-6 to 1.0×10-2mol/Lat 25 °C. The influence of membrane composition, pH of the test solutionand foreign ions on the electrode wereinvestigated. The electrode was successfully applied to determination of the drug in pure form, urine and serumby standard addition potentiometry.
KEYWORDS:Citapolarmhydrobromide(CT); Ion selective electrodes; potentiometry
Download this article as:| Copy the following to cite this article: Alturiqi A. S, Aljazzar S. O, Ammar R. A, khalaf N. Construction and Performance Characterization of Ion Selective Electrodes for Potentiometric Determination of Citapolarm Hydrobromide in Biological Fluids. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Alturiqi A. S, Aljazzar S. O, Ammar R. A, khalaf N. Construction and Performance Characterization of Ion Selective Electrodes for Potentiometric Determination of Citapolarm Hydrobromide in Biological Fluids. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=29535 |
Introduction
Citalopram hydrochloride(CT) (Fig. 1), (1RS)-1-[3-(Dimethylamino)propyl]-1-(4-fluorophenyl)-1,3-dihydroisobenzofuran-5-carbonitrile hydrobromide, is an antidepressant drug of the selective serotonin reuptake inhibitor (SSRI). The therapeutic mechanism of action of SSRIs involves the potentiation of serotonin [5-hydroxytryptamine (5-HT)] by the inhibition of its neuronal uptake. Serotonin is aneurotransmitter with neurons located in the raphe nuclei. Serotonergic neurons are known to play a part in sleep-wakefulness cycles, thermoregulation, mood, emotional and food behaviours. A meta-analysis, including studies with fluoxetine, paroxetine, sertraline, escitalopram, and citalopram versus placebo, showed SSRIs to be effective in reducing symptoms of premenstrual syndrome, whether taken continuously or just in the luteal phase1. Citalopram has produced a modest reduction in alcoholic drink intake and increase in drink-free days in studies of alcoholics, possibly by decreasing desire or reducing the reward2.Citalopram has been found to reduce the symptoms of diabetic neuropathy 3.Several methods have been reported for the determination of CT including spectrophotometry 4-8, capillary electrophoresis 9-11 gas chromatography 12, thin layer chromatography 13 and high performance liquid chromatography 14, 15.The present work describes preparation, characterization and application of coated wire electrode for continuous determination of CT in pure form and in biological fluids.
 |
Figure 1: Chemical structure of citapolarm hydrobromide. |
Experimental
Reagents and Materials
Citapolarm hydrobromide (CT) was provided by Sigma-Aldrich. Sodium tetraphenyl borate (NaTPB) (C6H5)4BNa, dioctylphthalate (DOP) C24H38O4 were obtained from Sigma-Aldrich. Poly(vinylchloride) (PVC) of high molecular mass and tetrahydrofuran(THF) were obtained from Fluka. Stock solution of CT (1.0×10-2mol/L) was prepared by dissolving the accurately weighed amount into a 100-mL volumetric flask, which was dissolved in sufficient amount of phosphate buffer pH (5.3), and then the volume was brought up to the mark with the same solvent. Working solutions of the drug (1.0×10-7to 1 × 10-2mol/L) ) were freshly prepared by serial dilutions from the CT stock solution using phosphate buffer (pH 5.3) as a solvent.
Instrumentation
All potentiometric measurements were carried out with an Orion (Cambridge, MA, USA) Model 701 A digital pH/mV-meter. Ag/AgCl electrode was used as an external reference electrode.
The electrochemical system for the membrane electrode is represented as follows: Copper/membrane/test solution//KCl salt bridge/Ag/AgCl
Preparation of ion-pair compound
The ion-pair was prepared by mixing 50 ml aliquots of 1.0 × 10-2 mol/LCT drug and sodium tetraphenyl borate. The resulting precipitate was filtered through G4 sintered glass crucible and washed thoroughly with deionized water, then dried at room temperature for 24 h. The ion-pair should be stored in a desiccator.The chemical composition of the ion-pair as identified by elemental analysis was found to be 1:1 (CT-TPB).
Electrode fabrication
The membranes of optimum composition were prepared by dissolving the different amounts ofion-pair, PVC and DOP in 5 ml THF.The solution was mixed well into a 5 cm diameter glass Petri dish, covered with a filter paper and left to stand overnight to allow solvent evaporation at room temperature.Copper-wire, about 1cm long and 1 mm in diameter with a spherical head, sealed into the end of a glass tube and soldered onto a shielded cable, was dipped into the membrane solution three times, and the solvent was evaporated each time at room temperature. A membrane wasformedonthecoppersurface;itwasallowedtosetfor2h. The electrode was then rinsed with water and finally conditioned by soaking in in 10−3 mol/L CT for 12 h before use.
Construction of the calibration graphs
The electrode was calibrated by separately transferring 50 mL aliquots of solutions ((1× 10-7 to 1× 10-2mol/L) of CT into a series of 100 mL beakers. The Prepared electrode in conjunction with the reference electrode was immersed in the above test solutions and allowed to equilibrate while stirring. The potential was recorded after stabilizing to ± 1 mV. The electrode was washed with phosphate buffer, pH 5.3 between measurements.The electrode potentials, Eelec, were calculated from the e.m.f. values and plotted versus negative logarithmic concentration of CT , Slopes of the resulting calibration curves were calculated.
Electrode selectivity
The selectivity coefficient values for the proposed electrodewere determined by the separate solution method17, in which the following equation was applied:
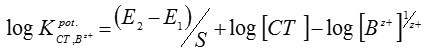
where E1 and E2 are the electrode potentials of solutions of the CT drug and interfering cation, Bz+, respectively (1×10–3mol/L of both atomoxetine and the interferent) and S is the slope of the calibration graph.
Potentiometric determination of citapolarmhydrobromide
CT was determined potentiometrically using the investigated electrode by the standard addition method 16. In the standard addition method, Small increments of a standard CT solution 1.0×10-2mol/L were added to 50 mL aliquot samples of various drug concentrations. The change in potential reading at a constant temperature of 25 ±1˚C was recorded for each increment and used to calculate the concentration of the drug sample solution.
Determination of citapolarmhydrobromide in biological fluids
Aliquots of 5 mLplasma or urine of a healthy person were placed in 50-ml measuringflasks, and different amounts of CT wereadded separately with constant shaking. The membraneelectrode was immersed in these solutions and potentiometricdetermination was carried out. The electrode was washed with water between measurements.
Results and Discussion
Composition of the membrane
Numerous membrane compositionsas indicated in Table 1 were investigated, the excellentperformance was obtained by using composition containing 13% CT-TPB, 43.5.0% of each PVC and DBP with resulting slope of 56.83 mV/decade over the concentration range of 1.0×10-5 to 1.0×10-2mol/L at 25°C. The above optimum composition was used to prepare membrane electrodes for all further subsequent investigations.
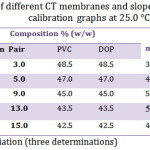 |
Table 1: Composition of different CT membranes and slopes of the corresponding calibration graphs at 25.0 °C. |
Life time
The electrode response time was examined for 1.0 × 10-4-1.0 × 10-2 mol/L drug solutions.The measurements sequence was ordered from low to high concentrations.The electrodeexhibited a fast and dynamic responseof 25 s for a period of 30 days, without significant change in theelectrode parameters.
Optimization of pH
The effect of pH of the CT test solution (1.0 × 10-4_1.0 × 10-2 mol/L) on the electrode potential was investigated by following the potential variation with change in pH by the addition of small amount of HCl and/or NaOH (0.1–1 mol/L of each). It was cleared that the electrode do not respond to pH changes in the range 3.4-7.8. At a pH less than 3.4, the response of electrode increased as the analyte acidity increase; the membrane may extract H+, leading to noisy responses. Because of the formation of non-protonated dimethyl amino group, the gradual decrease in potential noticed at a pH-value more than 7.8, which leads to a consequent decrease in its concentration.
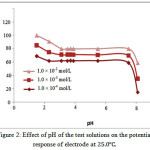 |
Figure 2: Effect of pH of the test solutions on the potential response of electrodeat 25.0. |
Selectivity measurements
The effect of different basic materials on the electrode response was detected by measuring the potentiometric interference from many inorganic cations, sugars, organic, and amino acids. The values of selectivity coefficient for the proposed electrode which shown in Table 2 were very small, this means that there is no interference of these cations with the response of CT electrode. Because of their ionic size, and consequently in their permeabilites and mobilities comparing to drug, the inorganic cations did not have any interfere. In the case of amino acids and sugars, the high selectivity is mainly attributed to the lipophilic nature of their molecules relative to CT cation and to the difference in polarity.
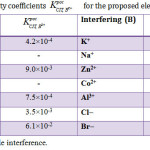 |
Table 2: Selectivity coefficients for the proposed electrode at 25.0 |
Regeneration of CT CWE
After working for a long time with the electrode, it becomes exhausted, so a regeneration procedure was done by soaking the exhausted electrode for 12 h in a solution of 1.0×10−3 mol/L CT solution. The slope was increasing from 53.7mV/decade to 56.3 mV/decade after regeneration. The reproducibility of repeated measurements on the same solutions was ±1 mV.
Analytical Applications
The proposed electrode was successfully employed for the determination of CT in pure solution, plasma and spiked urine by direct potentiometry using the standard addition method.The obtained average recovery and relative standard deviation values are summarized in Tables 3 and 4respectively, which reflect the high accuracy and precision of the electrode.
Table 3: Determination of citapolarm hydrobromide in pure solutions applying the standard addition method at 25.0 .
|
Added concentration (mg/ml) |
Recovery (%) |
RSD* |
|
2 |
100.0 |
1.18 |
|
3 |
102.5 |
0.40 |
|
5 |
99.8 |
1.66 |
|
Average recovery 100.77 |
||
*RSD (three determination)
Table 4: Determination of citapolarm hydrobromide in spiked plasma and urine samples applying the standard addition method at 25.0℃.
|
Spiked urine |
Spiked plasma |
|||
|
Added concentration (mg/ml) |
Recovery (%) |
RSD* |
Recovery (%) |
RSD* |
|
1.5 |
100.80 |
0.72 |
98.90 |
0.52 |
|
2.5 |
97.63 |
2.44 |
100.04 |
0.37 |
|
3.5 |
99.18 |
1.12 |
99.44 |
0.81 |
|
5 |
99.95 |
1.04 |
101.11 |
0.15 |
|
Average recovery |
99.39 |
99.87 |
||
* RSD (three determination)
Conclusion
A coated wire citapolarm-selective electrode based on incorporation of citapolarm- tetraphenylborate ion pair in a poly(vinylchloride) coating membrane was constructed and used for determining citapolarm HBr in pure form, urine and serum. The proposed ion-selective electrode has shown good performance characteristics with time stability up to five weeks. The good recoveries and low relative standard deviation reflect the high accuracy and precision of the proposed method. Moreover, the procedure is simple, easy to operate and it is inexpensive determination to make the electrode, therefore, an excellent tool for the routine determination of CT in quality control laboratories.
Acknowledgment
This research project was supported by a grant from the Deanship of Scientific Research. Princess Nourah Bint Abdul Rahman University.
References
- Marjoribanks, J.; Brown, J.; O’Brien, P.M.; Wyatt, K.; “Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev.2013
CrossRef - Tiihonen, J.; Ryynänen, O.P.; Kauhanen, J.; Hakola, H.P.; Salaspuro, M. Pharmacopsychiatry 1996, 29, 27–9.
CrossRef - Sindrup, S.H.; Bjerre, U.; Dejgaard, A.; Brøsen, K.;, Aaes-Jørgensen, T, Gram, L.F. Clin. Pharmacol. Ther. 1992, 52, 547–52.
CrossRef - Raza, A. Chem. Pharm. Bull. 2006,54, 432-434.
CrossRef - Darwish, I. A.; Refaat, I. H.; J. AOAC Int. 2006, 89, 326-333.
- Menegola, J.; Steppe, M.; Schapoval, E.E.S.; J. AOAC Int. 2008, 91, 52-58.
- Narayana, B.; Veena, K. J. Mex. Chem. Soc. 2010, 54, 98-102.
- Halvorsen, T. G.; Pedersen –Bjergaard, S.; Rasmusse, K. E. J. Chromatogr. A 2001, 909, 87-93.
CrossRef - Rong, Z.; Shangyou, X.; Hongmei, X.; Rui, H.; Zhining, X. Chin. j. Anal. Chem. 2006, 34, 1384-1388.
CrossRef - Nevado, J.J.B.; Cabanillas, C. G.; Llerena, M.J.V,; Robledo, V. R., J. Chromatogr. A 2005,1072, 249–257.
CrossRef - Berzas, J.J.; Guiberteau, C.; Villasenor, M.J.; Rodriguez, V. Anal. Chim. Acta , 2004,519, 219–230.
CrossRef - Saravanan, M.T.C.; Kumar, C.A.S.; Sudhakar, C.; Rajesh, B.; Kumar, G.S. JASR. 2012, 3, 62.
- Pistos, C.; Panderi, I.; Atta-Politou, J.; J. Chromatogr. B 2004, 810, 235-244.
CrossRef - Rao, R. N.; Raju, A. N.; Nagaraju, D. J. Pharm. Biomed. Anal. 2006, 41, 280-285.
CrossRef - Sujatha, K.; Rao, J. V. L. N. S. Pharmanest. 2013, 4,1299-1605.
- Baumann, E. Anal. Chim. Acta 1986, 42, 127-132.
CrossRef - Moffat, A.C.; Jackson,J.V.; Moss, M.S. ; Widdop,B. The pharmaceutical press: London,1986, 794.

This work is licensed under a Creative Commons Attribution 4.0 International License.









