Chemical Composition and Radical Scavenging Activity of Citrus Limon Peel Essential Oil
Mona Ghoorchibeigi1, Kambiz Larijani1, Parviz Aberoomand Azar1, Karim Zare1 and Iraj Mehregan2
1Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
2Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: larijani_k@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/330153
The water distillated essential oil of Citruslimon collected from Ramsar, Province of Mazandaran, North of Iran collected in December 2013, was analyzed using gas chromatography (GC) and gas chromatography-mass spectroscopy (GC-MS). The yield of oil was 0.23% w/w. Twenty-one components representing 100% of the essential oil were characterized. Limonene (61.4%), b-pinene (13.1%) and g-terpinene (11.3%) were identified as the main constituents in the volatile oil. The antioxidant ability of the oil was examined by free radical scavenging method using 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical at different concentration of the oil. The Citruslimon oil exhibited free-radical-scavenging properties with IC50 value of 284.71µg ml-1.
KEYWORDS:Citrus limon; essential oil; Radical Scavenging; DPPH
Download this article as:| Copy the following to cite this article: Ghoorchibeigi M, Larijani K, Azar P. A, Zare K, Mehregan I. Chemical Composition and Radical Scavenging Activity of Citrus Limon Peel Essential Oil. Orient J Chem 2017;33(1). |
| Copy the following to cite this URL: Ghoorchibeigi M, Larijani K, Azar P. A, Zare K, Mehregan I. Chemical Composition and Radical Scavenging Activity of Citrus Limon Peel Essential Oil. Orient J Chem 2017;33(1). Available from: http://www.orientjchem.org/?p=27840 |
Introduction
Essential oils are complex mixture of volatile compounds which consist of terpenes and their oxygenated derivatives such as aldehydes, alcohols and ketones with high potential bioactivity like antibacterial, antifungal and antioxidant that extracted mainly using steam and hydrodistillation (1, 2).
The genus Citrus which belonged to Rutaceae family is represented in Iran by several species such asC. sinensis, C. medica, C. limon, C. nobelis, C. aurantifolia and C. aurantium. Citrus fruits are the most common subtropical plants in the world with a numerous variation due to frequent bud mutation, interspecific and intergeneric hybridization. In Iran, the phylogeny of many citrus variants is remained unknown (3). In last decade, the essential oil of Citrus limon were the subject of previous study and limonene, a hydrocarbon monotrepene, is dominant constituents which shown sufficient antifungal activity (4). The oil of C. limon was shown acceptable activity against Anopheles stephensi (malaria agent) compare to N, Ndiethyl-3-methylbenzamide (Deet) as a standard synthetic repellent (5).
This study deals with the composition characterization and antioxidant activity using free radical scavenging method ofCitrus limon essential oil with cultivated in the North of Iran for the first time.
Material and Methods
Plant Material
The fruit of Citruslimon were harvested in from Ramsar, province of Mazandaran, North of Iran in December 2013. The collected material was identified in the Citrus Research Institute of Iran (Ramsar, Mazandaran).
Essential Oil Extraction
The Citruslimonfruits were washed with cold water and peeled. The peels of fruits were dried in shade and grinded. 100 gr of grinded peels were subjected to hydrodistillation using a Clevenger type apparatus for 3 h. The pale yellowish oil was dried over anhydrous Na2SO4 and kept in 4°C until analysis (6).
Qualitative Analysis of C. limon Oil Components
The qualitative analysis was done using a Hewlett-Packard 6890 GC coupled to a Hewlett-Packard 5973 mass selective detector equipped with a HP-5MS (30m × 0.25 mm, 0.32 µm film thickness) column. Oven temperature was programmed from 60°C (3 min) to 230°C at 6°C/min, and the final temperature kept for 3 min. split (1:30) injector temperature was 250°C. Carrier gas was He (99.999%) at 1 ml/min flow rate. The volume of injected sample was 1.0µl of diluted oil in hexane. The mass spectra were achieved at ionization energy 70eV, in the electronic ionization (EI) mode. Ion source temperature was 230°C. Scan mass range was adjusted of m/z 40-650.
The constituents of essential oil were characterized based on theirsimilarity of their mass spectra with those gathered in the Wiley library, or reported in the literature and their relative retention Index (RRI), calculated in relation to the retention time of a series of alkanes (C7– C20) as reference chemicals, in comparison with those of the chemical compounds gathered by Adams data (7)
Quantitative Analysis of C. limon Oil Components
The isolated oil was dissolved in n-hexane, and 1.0µl was injected to a Hewlett-Packard 6890 gas chromatograph equipped with HP-5 capillary column (30 m × 0.25 mm, film thickness 0.32 µm). The operating conditions were as follows: oven temperature program from 60°C (3 min) to 230°C at 6°C/min heating rate, kept for 3 min at the final temperature, split injection ratio 1:30, carrier gas nitrogen, flow rate 1mL/min, temperature of injector and detector (FID) fixed at 260°C and 280°C, respectively.
Antioxidant Activity of C. limonessential Oil
The stable organic radical DPPH˙has been widely used in the determination of the antioxidant activity of different plant extracts.This procedure is based on the reduction of DPPH˙solutions in the presence of plant extract. DPPH˙ solutions show a strong absorption band at 517 nm appearing a deep violet color. The ability of C. limon essential oil to quench reactive species by hydrogen (H+ ions) donation was measured through DPPH radical scavenging activity assay. Activity was measured as relative decrease absorbance at 517 nm as reaction between DPPH˙ and The oil. Antioxidant activity was evaluated with %50 (IC50) (8). A 2 ml of 0.1mM DPPH˙ methanol solution with 2 ml sample with 1, 5, 10, 50, 100, 200, 400, 600, 800 and 1000 µg ml-1 concentration with shaking. After the solution was incubated for 30 min at 25˚ C in dark, the decrease in the absorbance at 517nm was measured. Control contained methanol instead of sample solution. The radical scavenging activity percentage (%RSA) was calculated by the blow equation. Ascorbic acid (100 µg ml-1) was used as positive control.
%RSA= [(OD DPPH– OD sample)/OD DPPH] ×100
Result and Discussion
The pale yellowish essential oil of Citrus limon was obtain in the yield of 0.23 %w/w. The chemical composition of Citrus limonessential oil was listed in Table 1. Twenty one components, representing 100% of the total oil, were identified in Citrus limonessential oil. Limonene (61.4%), b-pinene (13.1%) and g-terpinene (11.3%) were the main constituents. The oil contained 94.1% hydrocarbon monoterpenes, 3.8% oxygenated monoterpenes, 2.0% hydrocarbon sesquiterpenes and 0.1% non-terpenes compounds.
Table 1: Chemical composition of Citrus limon essential oil
|
(%) |
KI |
Compounds |
|
0.1 |
900 |
Nonane |
|
0.6 |
930 |
a-thujene |
|
2.4 |
939 |
a-pinene |
|
2.3 |
975 |
sabinene |
|
13.1 |
979 |
b-pinene |
|
1.6 |
991 |
myrcene |
|
0.3 |
1017 |
a-terpinene |
|
61.4 |
1029 |
limonene |
|
0.3 |
1037 |
z-b-ocimene |
|
0.2 |
1050 |
E-b-ocimene |
|
11.3 |
1060 |
g-trpinene |
|
0.6 |
1089 |
terpinolene |
|
0.4 |
1188 |
a-terpineol |
|
1.1 |
1238 |
neral |
|
1.5 |
1267 |
geranial |
|
0.5 |
1362 |
neryl acetate |
|
0.3 |
1381 |
geranyl acetate |
|
0.4 |
1419 |
b-caryophyllene |
|
0.6 |
1435 |
E-a- bergamotene |
|
0.2 |
1500 |
bicyclogermacrene |
|
0.8 |
1506 |
b-bisabolene |
DPPH˙ radical scavenging activity
DPPH˙ radical-scavenging activity percentage (%RSA) was reported in Table 2. The values of DPPH˙ %RSA were presented in table 2 and the relation of extract concentration and %RSA was presented in figure 1.
Table 2: %RSA and absorbance of different concentration of Citruslimonessential oil
|
Oil concentration (µg ml-1) |
absorbance |
%RSA |
|
|
DPPH˙(blank) |
1.35 |
– |
|
|
1 |
0.93 |
31.11 |
|
|
5 |
0.92 |
31.85 |
|
|
10 |
0.91 |
32.59 |
|
|
50 |
0.80 |
40.74 |
|
|
100 |
0.81 |
40.00 |
|
|
200 |
0.76 |
43.70 |
|
|
400 |
0.61 |
54.81 |
|
|
600 |
0.47 |
65.18 |
|
|
800 |
0.26 |
80.74 |
|
|
1000 |
0.01 |
99.26 |
|
|
Positive control |
Ascorbic acid (100 µg ml-1) |
0.64 |
37.6 |
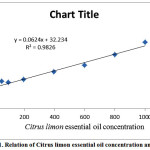 |
Figure 1: Relation of Citruslimon essential oil concentration and %RSA |
As shown in figure 3, the IC50 concentration could be calculated using equation of curve (y = 0.0624x + 32.234, R2= 0.9826) by replacing the amount of 50 instead of Y. The Citruslimon showed the IC50 at 284.71 µg ml-1. The amount of %RSA for the concentration of 100 µg ml-1 of essential oil and ascorbic acid (40.00 and 37.6%, respectively) were showed close free radical scavenging activity.
Conclusion
Demands for natural substance are increasing by food, cosmetic and medicine industry due to consumer’s needs. So the importance of studies on essential oils lies not only in the identification of their constituents but also in the possibility of linking the chemical contents with particular bioactive functional properties. The capacity of essential oils to prevent disease is an interest of researchers. There is a strong need to understand the preventive effect of essential oils for counter acting oxidative damages. Our studysuggested that essential oil of cituslimon peel can be considered as an auxiliary supplement.
Acknowledgment
The authors are grateful to Mr. NimaMohhamadi (ZakariaRazi Laboratory Complex, Science and Research Branch, Islamic Azad University, Tehran, Iran) for free radical scavenging tests.
References
- Mahalwal, V.S.; Ali, M. J. Essent. Oil Bearing Plants, 2003, 6, 31-35
CrossRef - Joshi, A.; Sharma, A.; R.K. Bachheti, R.K.; Pandey, D.P. Orient. J. Chem., 2016, 32, 331-340
CrossRef - Mozaffarian, V. A dictionary of Iranian plants names, FarhangMo’aser Publishers, Tehran, Iran, 1998
- Viuda-Martos, M.; Ruiz-Navajas, Y.;Fernández-López, J.; Pérez-Álvarez, J.Food Control,2008, 19, 1130–1138
CrossRef - Oshaghi, M.A.; Ghalandari, R.; Vatandoost, H.; Shayeghi, M.; Kamali-nejad, M.; Tourabi-Khaledi, H.; Abolhassani, M.; Hashemzade, M. Iranian J. Publ. Health, 2003, 32, 47-52.
- Al-Humaidi, J.; Orient. J. Chem., 2015,31, 2265-2270
CrossRef - Adams R P, Identification of EO components by gas chromatography/mass spectroscopy, Carol Stream IL: Allured Publishing Co. 2001
- Aghajani, Z.; Akhbari, M.; Esmaeili, B.Orient. J. Chem. 2014,30, 181-185
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









