Thermal Properties of Polyimide Composites with Nanostructured Silicon Carbide
Alyona Igorevna Wozniak, Vitaly Sergeyevich Ivanov, Olga Vladimirovna Kosova and Anton Sergeyevich Yegorov
Federal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances»(FSUE “IREA”).
Corresponding Author E-mail: egorov@irea.org.ru
DOI : http://dx.doi.org/10.13005/ojc/320616
A series of polyimide composites reinforced with different loadings of silicon carbide (SiC) nanoparticles are prepared by in-situ polymerization technique. The polyimide (PI) matrix resin is derived from 4,4’-oxydianiline (4,4’-ODA) and pyromelliticdianhydride (PMDA). The dispersions of SiC nanoparticles are prepared via ultrasonic irradiation or mechanical homogenization. In this method, the SiC nanoparticles are dispersed in diamine solution followed by polymerization with dianhydride. The composites obtained under sonication were found to have lower thermal properties than composites prepared under homogenization.
KEYWORDS:polymer composite; silicon carbide; polyimide; particle-reinforced nanocomposite; sonication; mechanical homogenization
Download this article as:| Copy the following to cite this article: Wozniak A. I, Ivanov V. S, Kosova O. V, Yegorov A. S. Thermal Properties of Polyimide Composites with Nanostructured Silicon Carbide . Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Wozniak A. I, Ivanov V. S, Kosova O. V, Yegorov A. S. Thermal Properties of Polyimide Composites with Nanostructured Silicon Carbide . Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=26289 |
Introduction
Aromatic polyimides (PIs) are known for their high thermal stability and chemical resistance as well as high mechanical properties [1]. Although there are many high temperature stable polyimides, there is a growing demand for the use of these materials at higher temperatures in oxidizing and corrosive environments.Thus, an oxidation-resistant materials are used to improve the properties of the polyimide composition [2].
Recent research directions have been focused on attempts to combine polymer structure and nanoparticles to obtain materials with high stiffness, strength and tribological properties [3]. This approach is based on the ability to improve and modify the properties of materials by adding nanofillers in a polymer matrix [4–7]. Aeronautics is one of the most promising fields in terms of composite materials consumption. In the aviation industry, metal or ceramic materials are widely replaced by fiber-reinforced polymer composites [8].
The SiC nanoparticles were chosen in this project because of their high chemical resistance, heat resistance, high thermal conductivity and outstanding mechanical properties.[9]. According to the literature,there are only one research group working in the field of PI/SiC composites [10, 11].The poly(triazole-imide)/SiCnanocomposites were prepared via in-situ polymerization. In comparison with pristine polymer matrix the poly(triazole-imide)/SiCnanocomposites had improved mechanical and thermal properties.
In previous work we introduced results of utilizing nanostructured silicon carbide particles as filler of poly-oxydiphenylene-pyromellitimide matrix [12]. In current paper we study thermal properties of PI composites in dependence of SiC loadings (0,1%, 0,5%, 1%, 2%, 3%, 4% by weight) and dispersion technique (ultrasonic or mechanical homogenizer). In this work we used matrix resin derived from 4,4’-oxydianiline (4,4’-ODA)and pyromelliticdianhydride (PMDA) (see Fig. 1).
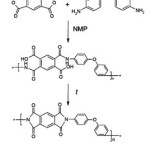 |
Figure 1: Synthesis of polyimide matrix |
Experimental Section
Materials
Nanostructured silicon carbide (SiC) was purchased from InKhimSintez. The properties of SiC are listed in Table 1.
Table 1: The properties of the SiC nanoparticles (according to InKhimSintez)
| Purity | > 97 % |
| Surface area | 30 – 35 m2/g |
| Color | From light gray to black |
| Morphology | Nearly spherical |
| Bulk density | < 0.5 g/cm3 |
Pyromelliticdianhydride (PMDA) was synthesized by oxidation of durene with potassium permanganate in pyridine followed by anhydridization with diphenyl ether according to the literature [13].
4,4’-Oxydianiline (4,4’-ODA) was purchased fromVekton and was purified prior to use by sublimation (~5 torr at ~155ºC).
1-Methyl-2-pyrrolidinone (NMP) was purchased from Component-Reaktiv and purified prior to use by drying over calcium hydride followed by distillation.
Preparation of the Nanocomposites
In a three-neck round-bottom flask equipped with a condenser and an argon inlet the mixture of 9,17 g (45,8 mmol) of 4,4’-ODA, SiC nanoparticles and 74 mL of NMP was dispersed by ultrasonic dispersant (at 20kHz upon cooling) or mechanical homogenizer (~4000 rpm) for 15 min. Then dispersant (or homogenizer) was replaced by mechanical stirrer and 10,0 g (45.8 mmol) of PMDA was added withsmall portions. The mixture was left stirring overnight and was stirred for another 20 hours. The resulting poly(amic acid) solution was cast onto clean and dry ceramic plates by a film applicator with thickness 500 μm. The films were dried in a vacuum oven at 80°C for 6 h.Then heating was performed in a programmed manner in the following sequence: one hour at 100ºC, one hour at 150ºC, one hour at 200ºC, one hour at 250ºC, one hour at 300ºC and 15 minat 350 °C.
Analytical Techniques
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) analysis was conducted under atmosphere of argon and air with a heating rate of 10ºC/minwith a TA Instruments SDT Q600. Scanning electron microscopy (SEM) was recorded with Hitachi SU-1510 VP-SEM under accelerating voltage of 7 kV and working distance of 5.5mm. IR-spectra were obtained on a VERTEX 70 FT-IR, Bruker Optics. Viscosities were measured with rheometer MCR 52, Anton Paar.
Results and Discussion
Synthesis of Nanocomposites
The nanostructured SiC was dispersed in solution of 4,4’-ODA in NMP under sonication or homogenization followed by polymerization with PMDA to give the poly(amic acid)s (PAA) with concentration of 20wt% with viscosities listed in Table 2. The experiments without loading of nanofiller were conducted to study the dependency of the viscosity of PAA solution from SiC content and dispersing method. PAA solutions with lower loadings of SiC and without nanofillerobtained under sonication were more viscous than PAA solutions obtained under homogenization that can be attributed to uniformly dispersed filler and/or greater values of molecular weights.
Table 2: Viscosities of PAA with different loadings of SiC, Pa·s
|
SiC content, wt% |
A dispersing method |
|
|
Sonication |
Homogenization |
|
|
0 |
7,86 |
5,94 |
|
0,1 |
10,10 |
4,79 |
|
0,5 |
11,30 |
4,01 |
|
1 |
2,89 |
3,97 |
|
2 |
5,24 |
7,13 |
|
3 |
5,06 |
6,82 |
|
4 |
4,70 |
4,19 |
Thermal imidization consists of casting the poly(amic acid) solution onto a suitablesubstrate, followed by gradual heating to effect solvent removal and the cyclodehydrationreaction to form polyimide.Solid films with color from beige to brown were obtained.
Properties of Composites
The infrared spectra of the films (Fig.2) were common for all composites and had absorption peaks specific to imide groups (1775 cm-1 (C=O), 1712 cm-1 (C=O), 1368 cm-1 (C-N), and 721 cm-1 (C=O). The absence of absorption band at 1660 cm-1(C=O amide) specific to poly(amic acid)shows complete imidization
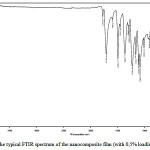 |
Figure 2: The typical FTIR spectrum of the nanocomposite film (with 0,5% loading of SiC) Click here to View figure |
The thermal properties of the polyimide nanocomposites were obtained by TGA. The 5% and 10% weight loss temperatures of films in air and inert atmosphere (Ar) are shown in Table 3. TGA/DSC curves of polyimide composites with 0,5%wt. and 4%wt. SiC (obtained under sonication or homogenization of nanofiller in diamine solution) in air are shown in Fig. 3 and Fig. 4. The sonication of monomer solution resulted in decrease of Td5 of pristine polyimide in air and inert atmosphere. The composites with 0,1% and 0,5%wt. SiC obtained under sonication also have lower thermal properties than composites prepared under homogenization of SiC in monomer solution.
Table 3: The thermal properties of the polyimide nanocomposites
|
SiC content, wt% |
A dispersing method |
Air |
Argon |
||
| Td/°C 5% weight loss |
Td/°C |
Td/°C |
Td/°C |
||
|
0 |
Sonication |
519 |
555 |
504 |
553 |
|
Homogenization |
540 |
567 |
566 |
579 |
|
|
0,1 |
Sonication |
518 |
557 |
557 |
577 |
|
Homogenization |
528 |
566 |
554 |
569 |
|
|
0,5 |
Sonication |
518 |
560 |
570 |
585 |
|
Homogenization |
539 |
569 |
562 |
576 |
|
|
1 |
Sonication |
557 |
578 |
556 |
573 |
|
Homogenization |
521 |
566 |
560 |
582 |
|
|
2 |
Sonication |
524 |
570 |
563 |
585 |
|
Homogenization |
531 |
567 |
552 |
573 |
|
|
3 |
Sonication |
496 |
541 |
558 |
577 |
|
Homogenization |
536 |
570 |
553 |
572 |
|
|
4 |
Sonication |
541 |
565 |
571 |
587 |
|
Homogenization |
513 |
554 |
569 |
585 |
|
 |
Figure 3: TGA/DSC curves of PI/0,5%SiC and PI/4%SiC composites (obtained under sonication of nanofiller in diamine solution) in air |
 |
Figure 4: TGA/DSC curves of PI/0,5%SiC and PI/4%SiC composites (obtained under homogenization of nanofiller in diamine solution) in air |
Solid films were examined by SEM to determine how the particles weredistributed in the polymer matrix (see Fig. 5). The nanofillerwas uniformly distributed in all concentrations. SEM microphotographs didn’t show any differences in composite surface depending on dispersing technique.
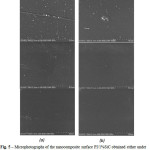 |
Figure 5: Microphotographs of the nanocomposite surface PI/1%SiC obtained either under sonication (a) or homogenization (b) of SiC nanoparticles in monomer solution |
Conclusion
A series of polyimide composite materials filled with nanostructured SiC with loadings from 0,1% to 4% wt. has been obtained by in-situ imidization and show good thermal stability. It was found that a method of dispersion of filler (sonication and homogenization) influenced on the thermal properties of composite. Sonication of monomer solution resulted in decrease of Td5 of pristine polyimide and PI/SiC composites compared to homogenization. Further research will be conducted for the detailed interpretation and understanding of current results. Further work on this project will focus on the production and study of composite materials with a variety of polyimides.
Acknowledgements
Applied researches are carried out with state financial support represented by the Ministry of Education of Russia under the Agreement on granting subsidies No.14.625.21.0003 of August 25, 2014. Unique identifier for Applied Scientific Researches (project) RFMEFI62514X0003.
References
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J-Y.; Ha, C.-S.Prog.Polym.Sci.2012, 37, 907– 974.
CrossRef - Morgan, A.B.; Putthanarat, S. Polym.Degrad. Stab.2011, 96, 23-32.
CrossRef - Zou, H.; Wu, S.; Shen, J. Chem. Rev. 2008, 108, 3893-3957.
CrossRef - Wang, P. J.; Lin, C. H.; Chang, S. L.; Shih, S. J. Polym. Chem. 2012, 3, 2867-2874.
CrossRef - Kwon, K.; Chang, J.-H.J. Compos. Mater.2015,49(24), 3031-3044.
CrossRef - Nemeryuk, A.M.; Lylina, M.M. Orient. J. Chem.2015, 31(4), 2481-2486.
CrossRef - Udmale, V.; Mishra, D.; Gadhave, R.; Pinjare, D.; Yamgar, R. Orient. J. Chem.2013, 29 (3), 927-936.
CrossRef - Trimble, S. Flight International 2011, 31/V-6/VI, 21-22.
- Guo, Z.; Kim, T.K.; Lei, K.; Pereira, T.; Sugar, J.G.; Hahn, H.T. Compos. Sci. Technol.2008, 68, 164-170.
CrossRef - Bazzar, M.; Ghaemy, M.; Alizadeh, R. Polym. Deg. Stab.2012, 97, 1690-1703.
CrossRef - Bazzar, M.; Ghaemy, M. Compos.Sci. Technol. 2013, 86, 101−108.
CrossRef - Wozniak, A.I; Ivanov, V.S.; Retivov, V.M.; Yegorov, A.S. Orient. J. Chem. 2015, 31(3), 1545-1550.
CrossRef - Yegorov, A.S., Ivanov, V.S., Wozniak, A.I. Biosci., Biotechnol. Res. Asia. 2014, 11(3), 1765-1779.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









