Synthesis and Characterization of Soy Lecithin Coated Magnetic Iron Oxide Nanoparticles for Magnetic Resonance Imaging Applications
Farzaneh hosseini and Mirabdullah Seyed Sadjadi
Department of Chemistry, Science and Research Branch, Islamic Azad University, Tehran, Iran.
Corresponding Author E-mail: m.s.sadjad@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320608
In this work, we report synthesis and characterization of soy lecithin (SL) coated iron oxide nanoparticles by one step co precipitation method in an aqueous solution using ferrous and ferric salts (1:2), different values of SLP (0.0, 1.5 and 6 gr) and ammonia to adjust pH=10. Characterization of the samples (labeled as SPION, SPION 1.5 and SPION6) carried out using X-ray powder diffraction (XRD) patterns indicated formation of magnetite (Fe3O4) nanoparticles with a calculated average crystalline size of 25 nm for naked Fe3O4, 13 nm for SION 1.5 and 9 nm for SION6 nanoparticles by Sherrer's equation. FT–IR spectroscopy and Thermo-gravimetric analysis (TGA) were used to investigate the presence of SL on the nanoparticles surface. The images and morphology of the samples were examined on scanning electron microscope (SEM). Detailed chemical analysis of the nanoparticles was obtained from energy dispersive X-ray (EDX) data. To measure magnetic properties of the prepared samples, a Vibrating Sample Magnetometer (VSM) was used and a Dynamic Light Scattering (DLS) instrument was finally used to measure hydrodynamic diameter of the nanoparticles. The results revealed that the soybean lecithin was coated on Fe3O4 nanoparticles surface and smaller particle size was obtained with increased concentrations of soybean lecithin.
KEYWORDS:Water dispersed solution; Soy Lecithin; Surface modification; Iron Oxide magnetic nanoparticles (SPION)
Download this article as:| Copy the following to cite this article: Hosseini F, Sadjadi M. S. Synthesis and Characterization of Soy Lecithin Coated Magnetic Iron Oxide Nanoparticles for Magnetic Resonance Imaging Applications. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Hosseini F, Sadjadi M. S. Synthesis and Characterization of Soy Lecithin Coated Magnetic Iron Oxide Nanoparticles for Magnetic Resonance Imaging Applications. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=26407 |
Introduction
Magnetic Iron oxide nanoparticles (SPION) with appropriate surface chemistry have been attracted a lot of interest for their numerous applications [1-3] such as magnetic resonance imaging contrast enhancement [4,5], immunoassay, detoxification of biological fluids, hyperthermia [6], drug delivery systems [7,8] and in cell separation and immobilization of biomolecules such as nucleic acids or proteins [9], etc. All of these biomedical applications require that these nanoparticles should have high magnetization values and size between 10 to 100 nm [10] with an overall narrow particle size distribution, so that the particles could have uniform physical and chemical properties and have to be optimized for intravenous injection and most prolonged drug circulation time in the body. Furthermore, magnetic nanoparticles need to have special surface coating to be not only non-toxic and biocompatible but also to allow a targetable delivery with particle localization in a specific area [11]. To this end, to prevent formation of the large aggregates and preparation of an appropriate substrate for functional groups (amines or carboxylic acid) for bio conjugation with certain drugs we attempted to synthesize in one pot soy lecithin protein coated iron oxide nanoparticles using in situ coating co-precipitation procedure. The size, morphology, and magnetization of the prepared samples as well as, the effect of different values of SL coating on the size and magnetic properties of magnetic nanoparticles will be finally reported
Materials and methods
Materials
Ferric chloride hexahydrate (FeCl3.6H2O) and Lecithin from Soybean were obtained from Sigma-Aldrich. Ferrous sulfate heptahydrate FeSO4.7H2O, and ammonium hydroxide 25 wt%, were purchased from Fluka (Buchs, Switzerland).
Methods
X-ray diffraction patterns (PW 1800 PHILIPS), and FT-IR spectra (Perkin Elmer spectrum 100) were used to determine crystal structure of SPION and its lecithin coated products. The morphology, particle size and particle size distribution determination were carried out using Scanning electron microscopy (Philips EM208) and Dynamic Light Scattering (Otsuka, LPA-3000, 3100, Japan) analysis. The presence of lecithin on SPION was studied by Thermogravimetric analysis (TGA 931 TA Perkin-Elmer instruments) and Eltra-cs analyzer. The magnetic properties were finally evaluated using a Vibration Sample Magnetometer (VSM, Quantum Design PPMS-9).
Preparation of SL Coated Iron Oxide Nanoparticles.
Soy lecithin coated iron oxide nanostructures were synthesized following a simple one-step coprecipitation approach. Initially, 2.7 g of ferric chloride hexahydrate FeCl3.6H2O and 1.39 g of ferrous sulphate heptahydrate FeSO4. 7H2O (molar ratio 2:1, respectively) were dissolved in 50 mL deionized water and the mixture, stirring vigorously under N2 atmosphere was heated to 85oC and was add drop wisely add 200 mL of ammonia solution (25%) containing 0.00, 1.50 and, 6.00 g of soy lecithin deoxygenated by dry nitrogen stirring over a period of 20 min until observing change of color from dark orange to black. Stirring was steadily continued for more than 2 h followed under rapid stirring in N2 atmosphere. The precipitates were finally removed by magnetic decantation and washed for several times with water to make it free of any residual salts, until obtaining pH= 7. The final products were dried in a vacuum oven at room temperature for 24h.
Preparation of water dispersed solution of SL Coated Iron Oxide Nanoparticles
In order to obtain good hydration of lecithin coated iron oxide nanoparticles, considering that lecithin is a mixture of phospholipids with phosphatidylcholine (PC) as a main component (up to 98% w/w), we prepared a dispersed aqueous solution of lecithin coated iron oxide nanoparticles by dispersing our synthesized lecithin coated iron oxide nanoparticles (SPION6) in water or in an isotonic aqueous solution by means of extensive mixing at temperature 40–60°C.
Results and discussion
X-ray power diffraction study
Fig. 1 represents XRD patterns of SPION6 (a); SPION1.5 (b) and naked Fe3O4 (c) nanoparticles. This results show clearly that, all the patterns are in good agreement with the standard diffraction spectrum (JCPDS Card No. 19-0629) [12] and the synthesized products were crystalline Fe3O4. The average particle size was calculated to be at about 25 nm for naked Fe3O4, 13 nm for SPION1.5 and 9 nm for SION6 nanoparticles by using Sherrer’s equation:
D = Kλ/(βCosθ)
Where, D is the particle size and K, λ, β and θ denote Sherrer constant, X- ray wavelength, the peak width of half-maximum and the Bragg angle. These results and comparing the crystalline size obtained indicate that, SLP has served as a surfactant and coating agent in the precipitation of Fe3O4 nanoparticles and we found that, the size of coated Fe3O4 nanoparticles prepared by this method was decreased by increasing concentration of soy lecithin. This phenomena can be related to the nature of soy lecithin which is a mixture of phospholipids with phosphatidylcholine (PC) as a main component (up to 98% w/w). Noting that, phosphatidyl cholines molecules are amphiphatic (both hydrophilic and hydrophobic), and in accordance with other authors [13], we can conclude that, the SL in aqueous environment can make bilayer structure containing an aqueous phase entrapped by lipid bilayers, [14] labled as vesicles and can be encapsulate a region of aqueous solution containing ferrous, ferric and hydroxide ions within a hydrophobic membrane. As a result, iron oxide nanoparticles were formed in the limited size in the liposomes vesicles.
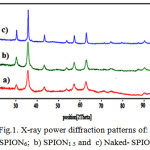 |
Figure 1: X-ray power diffraction patterns of: a) SPION6; b) SPION1.5 and c) Naked- SPION |
FTIR study
Fourier transform infrared spectroscopy can be used in order to identify the soy lecithin presence on Fe3O4 nanoparticles surface. The FT-IR spectra of the free soy lecithin (a), soy lecithin coated iron oxide nanoparticles (SPION)6 (b) and Naked (SPION) (c) are given in Fig.2. In this figure, naked iron oxide nanoparticles indicate strong band at 571 cm−1 that is assigned to the stretch vibration mode of Fe−O bond. Similar peaks have been observed in the spectrum of the soy lecithin coated iron oxide nanoparticles (SPION) at 580 cm-1. The formation of soy lecithin coating on the surface nanoparticles was confirmed through the comparison of soy lecithin and SPION spectra (Table 1). Other additional bands observed peaks at 3710 cm-1 and 3400 cm-1, which indicated the presence of N-H stretching and O-H stretching of amino group and hydroxyl group as well as the bands at about 1245 attributed to the presence of P=O (phosphomoyl) group [15] and appearance of a new broad band at about 2400 cm-1 in soy lecithin coated SPION’s spectrum can be clearly attributed to the presence of soy lecithin coats on the SPION nanoparticles.
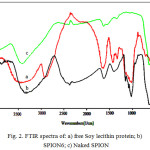 |
Figure 2: FTIR spectra of: a) free Soy lecithin protein; b) SPION6; c) Naked SPION |
Table 1: FTIR band assignment for naked fe3O4, SION6 and free Soy lecithin
| Bond |
Naked Fe3O4 |
SION |
Soy Lecithin |
| υ (Fe-O) |
571 |
580 |
|
| υ (HO-H) stretching |
3412 |
||
| υ (C=O) |
1624 |
1620 |
|
| υ (P-O) |
1075 |
1075 |
|
| υ (C-H ,CH2 and CH3) |
1322-1400 |
1331-1427 |
|
| υ (C-O-C) |
1153 |
1160 |
|
| υ(choline-containing phospholipids) |
1020 |
1020 |
Magnetic measurements
In order to study nanoparticles magnetic behavior and the effect of soy lecithin coating in magnetic properties, magnetization measurements for naked Fe3O4 and soy lecithin coated Fe3O4 nanoparticles ( SPiN 1.5 and SPION 6) were performed. As can be observed Fig.3, all of them have a hysteresis loop with zero coercivity and remanence values. This means that these are single domains with superparamagnetic characteristics. The saturation magnetization value of naked Fe3O4, SPION 1.5 and SPION6 were found to be 56.8, 48.5 and 31.3 electromagnetic units per gram (emu/g) respectively. The reduction in saturation magnetization was likely due to the existence of soy lecithin on the surface of Fe3O4 nanoparticles. This reduction is in direct relation with the soy lecithin coating density. Nanoparticles with a denser coating, which have been synthesized in higher soy lecithin concentrations, are less sensitive to a magnetic field. [16]
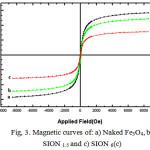 |
Figure 3: Magnetic curves of: a) Naked Fe3O4,b), SION 1.5 and c) SION 6(c) |
Scanning Electron Microscopy
The morphology of the synthesized was investigated using Scanning electron microscope. The SEM photograph of prepared samples, naked Fe3O4, SNIO1.5 and SNIO6 is shown in Figure 4. SEM image shows that the morphology of the Fe3O4 nanoparticles is roughly spherical shape. spherical shapes are usually formed because the nucleation rate per unit area is isotopic at the interface between the Fe3O4 magnetic nanoparticles [17], which the driving force for Ostwald ripening, minimization of the surface free energy by reduction of total surface area/volume, results in the equivalent growth rate along different directions of the nucleation because the sphere has the smallest surface area per unit volume of any shape [18].
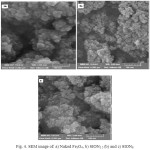 |
Figure 4: SEM image of: a) Naked Fe3O4, b) SION1.5 (b) and c) SION6 |
Thermo Gravimetric analysis
Thermogravimetric analysis (TGA) of naked and soy lecithin coated iron oxide nanoparticles were presented in Figure 5. This analysis was carried out for powder samples (7 mg) in N2 atmosphere with a heating rate 10 0C/min from 100 up to 700 oC. For naked Fe3O4, no significant peak appeared in the TGA curve. Also, it indicates that the weight loss in the temperature ranged from 100 to 300 only around 2%. This may be justified by the loss of water remaining on surface of iron oxide nanoparticles. For soy lecithin coated iron oxide, the TGA curve shows gradual and steady weight loss in the temperature ranged from 200 to 430 oC results in the burning of soy lecithin coated. As it is shown in the figure, the further soy lecithin concentration, the greater amount of weight loss is observable.
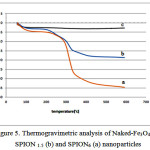 |
Figure 5: Thermogravimetric analysis of Naked-Fe3O4(c), SPION 1.5 (b) and SPION6 (a) nanoparticles |
Dispersive X-ray study
Fig. 6 and Table.2 represent the data obtained using X-ray energy dispersion (EDX) and Eltra-cs analyzer for as prepared samples, SPION1.5 and SPION6 . These results confirm the presence of iron (Fe), oxygen (O) with iron abundance higher than oxygen. Eltra-cs analyzer was used to confirm the presence of soy lecithin on surface of Fe3O4 nanoparticles by the analysis of carbon. The results indicate that percentage of carbon in SPION6 (19.35) is more than SPION1.5. (7.82). This results shows that, the amount of adsorbed soy lecithin on the nanoparticle surface is higher when we used higher soy lecithin concentration. This results will be confirmed by the thermo-grd avimetry analysis Spectrum.
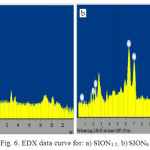 |
Figure 6: EDX data curve for: a) SION1.5; b)SION6 |
Table 2: EDX and eltra-cs analysis data for SION 1.5 and SION 6 nanoparticles
| Element | Line | Mass[%] | Atomic[%] | Formula | Mass[%] | |
| SION1.5 |
Fe |
K |
66.62 |
33.89 |
Fe3O4 |
90.68 |
|
O |
25.93 |
46.75 |
||||
|
C |
7.82 |
18.78 |
7.82 |
|||
| SION6 |
Fe |
K |
57.81 |
25.47 |
Fe3O4 |
79.89 |
|
O |
22.53 |
34.65 |
||||
|
C |
19.35 |
39.64 |
19.35 |
Dynamic Light Scattering (DLS) study
The DLS measurements results of naked Fe3O4 (a), SPION 1.5 (b) and SPION6 (c) dispersed in aqueous solution are shown in fig. 7. This figure shows that the hydrodynamic diameter for 25 nm particles is 78 nm while for 13 and 9 nm particles, prepared by increased soy lecithin concentration, reveal higher hydrodynamic diameter (342 and 955nm respectively). This results confirms increasing hydrodynamic diameter of the sample by increasing soy lecithin concentration.
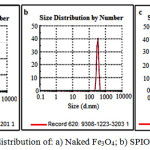 |
Figure 7: Particle size distribution of: a) Naked Fe3O4; b) SPION1.5 and c) SPION6 |
Conclusion
In summary, soy lecithin coated iron oxide nanoparticles (SPION) were successfully synthesized by in situ co-precipitation method. By increasing soy lecithin concentration in the preparation process, the SPION crystalline size decreased from 25 to 9 nm. The presence of soy lecithin on the surface of iron oxide nanoparticles has been confirmed through XRD patterns and FTIR spectra respectively. The roughly spherical morphology of the soy lecithin coated SPION confirms that soy lecithin in aqueous environment has formed bilayer structures ferrous, ferric and hydroxide ions within a hydrophobic membrane. As a result, iron oxide nanoparticles were therefore formed in the limited size in the liposomes vesicles. Investigation performed by TGA and DLS analysis were finally confirmed that, SPION nanoparticles surface was protected by soy lecithin layers as a surfactant.
References
- Yan Wei, Bing Han, Xiaoyang Hu, Yuanhua Lin, Xinzhi Wang, Xuliang Deng Synthesis of Fe3O4 nanoparticles and their magnetic properties. Procedia Engineering 2012 ; 27: 632 – 637.
CrossRef - Yulei Tai, Li Wang , Guangqing Yan, Jin-min Gao, Haojie Yu and Lei Zhang, Recent research progress on the preparation and application of magnetic nanospheres. Polymer International 2011; 60: 976–994.
CrossRef - J. Xie, J. Huang, X. Li, S. Sun and X. Chen., Iron oxide nanoparticle platform for biomedical applications. Curr Med Chem 2009; 16: 1278–1294.
CrossRef - Hyon Bin Na,In Chan Song, Taeghwan Hyeon. Inorganic Nanoparticles for MRI Contrast Agent Adv. Mater., 2009; 21:2133–2148.
CrossRef - Derong Zhu, Fuyao Liu, Lina Ma, Dianjun Liu, and Zhenxin Wang. Nanoparticle-Based Systems for T1-Weighted Magnetic Resonance Imaging Contrast Agents. Int J Mol Sci 2013 ;14(5): 10591–10607
CrossRef - Andreas Jordan, Regina Scholz , Peter Wust, Horst FaKhling , Roland Felix Magnetic fuid hyperthermia (MFH): Cancer treatment with AC magnetic “eld induced excitation of biocompatible superparamagnetic nanoparticles. Journal of Magnetism and Magnetic Materials 1999; 201:413-419.
CrossRef - Liu-Jiang Zhou, Bo Heand Feng. Zhang. Facile One-Pot Synthesis of Iron Oxide Nanoparticles Cross-linked Magnetic Poly(vinyl alcohol) Gel Beads for Drug Delivery, ACS Appl. Mater. Interfaces, 2012; 4: 192–199.
CrossRef - Fahima Dilnawaz1, Abhalaxmi Singh1, Chandana Mohanty , Sanjeeb K. Sahoo. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials, 2010;31:3694–3706.
CrossRef - Albert Figuerola, Riccardo Di Corato, Liberato Manna, Teresa Pellegrino. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacological Research 2010; 62: 126–143.
CrossRef - Gert Storm, Sheila 0. Belliot, Toos Daemenb, Danilo D. Lasic. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Advanced Drug Delivery Reviews 1995; 17: 31-48.
CrossRef - Sophie Laurent, Delphine Forge, Marc Port, Alain Roch, Caroline Robic, Luce Vander Elst, andRobert N. Muller.Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008,;108: 2064–2110 .
CrossRef - Bragg,W.H. (1915) The structure of magnetite and the … Nature, 1915, 95: 561
CrossRef - Theresa M. Allena , Pieter R. Cullisb.Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews 2013; 65(1): 36–48.
CrossRef - Scholfield, C.R. (October 1981), “Composition of Soybean Lecithin”, Journal of the American Oil Chemists’ Society, 58 (10): 889–892, doi:10.1007/bf02659652, retrieved 2014.,08-21 – via USDA
CrossRef - Choudhary N., Pardhi D., Bhoyar M, Isolation of soy lecithin from soy sludge, Its standardization and Behavioral study, Asian J Pharm Clin Res, 2013 ., 6, 2, , 133-136.
- Ge Y, Zhang Y, Xia J, et al. Effect of surface charge and agglomerate degree of magnetic iron oxide nanoparticles on KB cellular uptake in vitro. Colloid Surf B Biointerfaces. 2009.,73 (2):294–301.
CrossRef - Yan Wei, Bing Han, Xiaoyang Hu, Yuanhua Lin, , , Xinzhi Wang, Xuliang Deng. Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties . Procedia Engineering, 2012.,27.,632-637.
CrossRef - Wensheng Lu, Yuhua Shen, Anjian Xie, Weiqiang Zhang.Green synthesis and characterization of superparamagnetic Fe3O4 nanoparticles. Journal of Magnetism and Magnetic Materials 2010., 322 .,1828–1833.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









