Study of the Nuclear Magnetic Resonance of Ni/Al + tox O2/Ni Nanoscale Thin Films
Mehdi Soodmand*
Department of Physics, Payame Noor University, Iran.
Corresponding Author E-mail: soodmand@mailfa.com
DOI : http://dx.doi.org/10.13005/ojc/320645
In this paper, Thenuclear magnetic resistance study of evaporated Ni / Al + tox O2 / Ni thin films is investigated duo to the potential application in industry because of their tunneling magnetoresistive characteristics. Results showed that the nuclear magnetic resistance of this metal-insulator-metal thin film structure is useful in determining several important fabrication parameters, such as the Ni crystal structure and the optimal O2 plasma oxidation time ox of the Al spacer layer.
KEYWORDS:Magnetic Resonance; Thin film; MIM structures
Download this article as:| Copy the following to cite this article: Soodmand M. Study of the Nuclear Magnetic Resonance of Ni/Al + tox O2/Ni Nanoscale Thin Films. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Soodmand M. Study of the Nuclear Magnetic Resonance of Ni/Al + tox O2/Ni Nanoscale Thin Films. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=26322 |
Introduction
In recent years, the tunneling magneto resistance effect have been studied by many researchers. This effect can be found in multilayer metal-insulator-metal nanostructures in which a current flow from one ferromagnetic electrode across an insulating barrier to another ferromagnetic electrode in a so-called tunnel junction [1-3]. In 1975, phenomena of a tunneling magnetoresistance effect for first time reported.Also, scientists developed the production of magnetic tunnel junctions with successful and reproducible methods [4-5].In the case of the characteristics of a junction, the material of the insulating barrier and magnetic layers is very important.The highest magneto resistance effect can be achieved with very thin film of aluminum oxide in order of ten angstroms. One of the other important fabrication parameter is that the all thin films must be flat, because to avoid any direct contacts between metal electrodes together [6]. Also, the oxidation time of aluminum layer are very important that which has a positive or negative effect on the tunneling magneto resistance. However, the oxidation method within optimal time is unique for this application. The nuclear magnetic resistance can be used in different applications, such as spectroscopy of the X-ray photoemission, rutherford backscattering and auger electron. In this article, we study the nuclear magnetic resistance of Ni / Al + tox O2/ Ni nanoscale thin film.
Experimental
The thin films used in this article have been fabricated at the Nanoyam research and development center by evaporation. For lowest noise ratio of the nuclear magneto resistance, we have grown 5×15 mm2 without using the shadow mask because for the sensitivity reasons. The thickness of the layers has the following details: 100 angstrom nickel, 50 angstrom alumina and 120 angstrom nickel. The SiO2 layers used as the substrates and were grown on 5×15 mm2. On the nickel bottom layer, and the aluminum layer have been evaporated at cryogenic temperatures. We used DC glow-discharge in an O2 pressure for the oxidation of the aluminum layer.
Results and discussion
In Fig.1, the nuclear magnetic resistance of nickel is shown. In this curve, can be seen a peak around 196 MHz. The intensity of the peak arises from Ni atoms with only Ni nearest neighbors and for lower frequencies below 195 MHz, this part of curve related to the interface signal arises from Ni atoms at the bottom and top interfaces. So, the balk pick indicating a polycrystalline nature of the Ni. Also, according to the importance of the interface roughness, we can estimate by evaluating the relative parts in the interface and bulk spectral range of the spectrum. This work can be done by fitting the peak with a Gaussian distributed line, which would have allowed for an analysis of the interface region in terms of different nearest neighbor atoms and configurations. Therefore, in our opinion most of the intensity in the interface region of the spectrum is due to Nickel-silicide formation at the bottom interface.
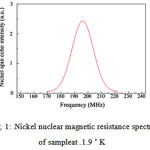 |
Figure 1: 59Nickel nuclear magnetic resistance spectrum of sampleat T = 1.9 ˚ K |
Fig.2, shows the nuclear magnetics resonance spectra of + the sample for various oxidation time 25, 50, 100 and 200 s. The spectra are normalized to a Nickel thickness.
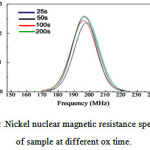 |
Figure 2: 59 Nickel nuclear magnetic resistance spectrum of sample at different on time. |
According to Fig.2, for the various oxidation time, all the curves are similar in shape (Gaussian form) and only there are some minor differences in the intensity distributions of the main peak. It is clear that no main changes are presented in the spin-echo spectra as function of the oxidation time. Therefore, we expect that antiferromagnetic properties is also present in our sample for oxidation times longer than about 100 s, although we have seen no changes in the nuclear magneto resistance spectra indicating its presence. In general, the hyperfine field at the nucleus site can be separated in a thermal avarage, responsible for the resonance frequency shifts, and a fluctuating part which induces random rotations or spin-flips of the nuclear moments, responsible for the relaxation phenomena. Fluctuations are also induced via the thermal motion of the electronic moment of the Ni, which is strongly coupled to the hyperfine field via the Fermi-contact interaction. If the fluctuations of the magnetic moment of the Ni are reduced, this results in an increase of the relaxation times. A common observation is that, when an external field is applied, which pins the magnetization in a certain direction and removes domain walls, the spin-spin and spin-lattice relaxation time dramatically increase. The spin-spin relaxation time is lower for the bulk resonance frequencies than for the interface parts, probably because there are less Ni atoms at the interfaces and hence less relaxation possibilities than for the bulk. The optimal oxidation time of 150 s, inferred from these results, is in perfect agreement with the resistivity measurements. Finally, it should be mentioned that the ferromagnetic enhancement factor decreases dramatically for larger oxidation times.
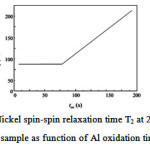 |
Figure 3: 59 Nickel spin-spin relaxation time T2 at 280 MHz of sample as function of Al oxidation time. |
The spin-spin relaxation time was measured with the CPMG sequence. The spin-spin relaxation time is lower for the bulk resonance frequencies than for the interface parts, probably because there are less Niatoms at the interfaces and hence less relaxation possibilities than for the bulk. However, this has not been systematically studied and verified. In the case of the Al nuclear magnetic resistance,the progressive oxidation of the Al spacer layer with oxidation time can be showed by nuclear magnetic resistance. Aluminum is paramagnetic and can therefore be distinguished by its Knight shift of 0.162 % from diamagnetic AlO x with zero Knight shift. However, because Al is not ferromagnetic, no enhancement of the resonance signals, like for Ni, occurs. We have measured Aluminum oxidized for 80 s, confined within 180 angstromNi. According to the Fig.4, two distinct lines are observed, related to Al and AlO x. The Al Knight shift of 0.26 % compares well to previous studies [3-6]. However, the signal to noise ratio is poor and does not allow for a quantitative interpretation of the data. Nevertheless, some qualitative information can be obtained from this spectrum. Also, Fig. 4, shows thatthe oxidation process is layer by layer, because two separate peaks are observed for Al and AlOx, rather than one broad distribution.
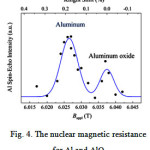 |
Figure 4: The nuclear magnetic resistance for Al and AlO. |
Conclusions
Results showed the structural nuclear magnetic resonance of Ni /Al + tox O2 /Ni thin films. Nickelnuclear magnetic resonancepresents that the Ni layers are not single crystalline, but include of a combine of fcc and hcp Ni. The Ni is oxidized according to the spin-spin relaxation time, when the Al spacer layer is exposed to oxygen longer than 150 s. Aluminum nuclear magnetic resonance shows two distinct lines of Al and AlO x, dipole broadened by stray fields emerging from the Ni layers, which might be related to the magnetostatic coupling between the two ferromagnetic layers.
References
- Margulies,D. T.; Parker, F. T.; Rudee, M. L.; Spada, F. E.; Chapman, J. N.; Aitchison, P. R.; Berkowitz, A. E. Phys. Rev. Lett. 1997, 79, 5162.
CrossRef - Gaines,J. M.; Bloemen, P. J. H.; Kohlhepp, J. T.; Bulle-Lieuwma, C. W. T; Wolf, R. M.; Reinders, A.; Jungblut, R. M.;Van Der Heijden, P. A. A.;Van Eemeren, J. T. W. M.; Stegge, J. aan de;De Jonge, W. J. M.,Surf. Sci.1997, 373, 85.
CrossRef - Voogt, F. C.; Palstra, T. T. M.; Niesen, L.; Rogujanu, O. C.; James, M. A.; Hibma, T.;Phys. Rev. B. 1998.57, R8107.
- Parkin, S. S. P.; Sigsbee, R.; Felici, R.; Felcher, G. P.;Appl. Phys. Lett. 1986, 48, 604.
CrossRef - Bashiz, R. T.; Shabanzadeh, E.; Orient. J. Chem.2015, 31(4), 2359-2368.
CrossRef - Jagadeesh, N.; Ravindrakumar, Y.; Mohanty, S.; Srinivasarao, T.; Jayashree, A.;Orient. J. Chem.2015, 31(3). 1801-1809.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









