Silver Nanoparticles Modification of Ultra High Molecular Weight Polyethylene in Non-Aqueous Medium
V. N. Glushko1*, L. I. Blokhina1, E. E. Anisimova1, M. V. Bogdanovskaya1, V. I. Kozhukhov1 and T. A. Cherdyntseva2
1Federal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances» (FSUE «IREA»), Bogorodskiyval, 3, 107076, Moscow, 107076, Russian Federation.
2Department of Microbiology, Faculty of Biology, M.V. Lomonosov Moscow State University, Leninskie Gory, 1-12, 119991, Moscow, Russian Federation.
Corresponding Author E-mail: tetrazoli@yandex.ru
DOI : http://dx.doi.org/10.13005/ojc/320630
A series of experiments for obtaining modified with silver nanoparticles ultra-high molecular weight polyethylene (UHMWPE) is done. Optimal precursors are silver trifluoroacetate, silver nitrate and silver methanesulfonate. Three variants of UHMWPE modification is studied: 1) the polyol synthesis, 2) polymer processing silver nanoparticle colloid and 3) reduction of silver salt solution in the UHMWPE polymer matrix. It is found that the last method is optimal. The specific surface of obtained nanoparticles is 7.1-7.6 m2/g and the size varies in the range of 60-110 nm. The antibacterial activity of the composite samples to E. Coliand S. Aureus is studied. Inhibition of bacterial growth occurs at the level 104cfu/ml and 106cfu/mlrespectively.
KEYWORDS:silver nanoparticles; UHMWPE; polyol synthesis; reduction; silver trifluoroacetate; silver methanesulfonate; silver laurate; silver palmitate; antibacterial activity
Download this article as:| Copy the following to cite this article: Glushko V. N, Blokhina L. I, Anisimova E. E, Bogdanovskaya M. V, Kozhukhov V. I, Cherdyntseva T. A. Silver Nanoparticles Modification of Ultra High Molecular Weight Polyethylene in Non-Aqueous Medium. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Glushko V. N, Blokhina L. I, Anisimova E. E, Bogdanovskaya M. V, Kozhukhov V. I, Cherdyntseva T. A. Silver Nanoparticles Modification of Ultra High Molecular Weight Polyethylene in Non-Aqueous Medium. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=25705 |
Introduction
One of the objects of modern medicine is the creation and improvement of materials for joint implant production (coxas, knee joints, spinal bones, dentofacial joints) for improvement their tribological and antimicrobic properties. Nowadays due to a number of properties (low constant of friction, high wear resistance and high chemical resistance) [1] theultra-high-molecular-weight polyethyleneholds a leading position as a material for prosthetics. There is number of works in literature devoted to the modification of UHMWPE with the metal nanoparticles, where the great attention is paid to introduction the silver nanoparticles into the polymer because of their unique antibacterial properties to the modification gains and UHMWPE. [2]The modified materialswithsilver nanoparticles have been already successfully used for the filter production and they areallowed to harmless pathogens, contained in the water for creation the special fabrics, paints and varnishes, which have antiseptic properties [3]. At the moment, there is a number of works where the polymers modified with the silver nanoparticles are used to create a new generation of implants and prostheses [4].
The improvement of the earlier known methods andthe development of such methods of nanomaterials producing identified main requirements that they must correspond:
to provide theobtainof controlled composition materials with reproducible properties;
to provide the temporal stability of nanomaterials, primarily provide the surface protection against spontaneous oxidation and sintering in the manufacturing process;
to have high production and economical efficiency;
to provide the obtain of nanomaterialswith a specific particle size or the obtain of grains which size distribution must be sufficiently narrow, if necessary.
In this work we proposed a method of modified UHMWPE with the silver nanoparticles, which corresponds these requirements. The numberof silver salts of organic acids and silver nitrate is used as precursors.
Theoretical Analysis
Up to date the developed methodsof nanocomposites obtaining are very diverse. The most common among the chemical and physical methods are listed below.
Inorganic nanoparticles and polymer are synthesized separately and then the nanoparticles are dispersed in the polymer (in its solution or melt). These methods include the dispersion in polymers of coarse particles with the colloid mills, ultrasound, high-frequency or arc crushing [5].
Inorganic particles and polymer are synthesized separately, and after that the polymer is grafted to the inorganic core. An example of such synthesis is the nanoparticles microencapsulation by various polymers when applied surfactant on the particle, and then polymer grafts to the surfactant molecule [6].
Inorganic nanoparticles which are obtained preliminary, serve as centers of heterophase polymerization of organic monomer. [7]
Inorganic nanoparticles are synthesized in situ in the polymer solution. By this strategyvarious chemicals belong, including photochemical and thermal methods of nanoparticles synthesis in polymers (recovery precursors, the decomposition of metal carbonyls in polymer matrices, etc.). [8].
Synthesis of inorganic nanostructures is combined with the polymerization of the organic monomer (creation of organic-inorganic copolymers based on organometallic compounds that are compatible with organic monomers) [9].
One of the main tasks in the developing polymer nanocomposites sphere is studying the nanoparticles formation processes in situ in a polymer matrix. There are various methods for nanoparticles forming in a polymer matrix, such as chemical synthesis methods in an organic solvent, [10], vacuum precipitating on theviscous polymeric materials [11], with a plasma polymerization with the metalspatter [12].
An important obtain aspect of the composite polymeric materials modified with nanoparticles, is the choice ofintroducing nanoparticles technology into the polymer structure.This also applies to the obtain of polymeric materials modified with silver nanoparticles.However, it is known that due to the strong hydrophobicity of UHMWPE the introduction of silver in its polymer matrix is complicated. Therefore, the preferred method of introducing silver to UHMWPE is dispersion in organic solvents.
To starting precursors for the impregnation of the polyethylene must correspond the certain requirements. First, they must have a solubility which is necessary to produce the desired concentration in the chosen solvent type, and a sufficiently high chemical stability under the chosen conditions.
As the precursors they selected: halogenated acetic acid salt (silver trifluoroacetate), sulfonic acid (silver methanesulfonate) salts of fatty acids (laurate, palmitate, and silver), and also the most used for this purpose – the silver nitrate.
For the silver nanoparticles modification of UHMWPE there were selected following methods from the literature:
Polymer impregnating with colloidal solution of silver nanoparticles [13];
Synthesis of silver nanoparticles in the polymer matrix:
a) with agentpresence [13];
b) polyol synthesis [14-17].
An ascorbic acid was selected as the reducing agent, and its effectiveness has been confirmed in previous works [18, 19].
Experimental Part
Reagents and solvents
They used powdered UHMWPE of Ticona company (GUR-4120) with the molecular mass of 5.0 million g/mol.; silver nitrate – 99.9%,trifluoroacetic acid – 99%,methanesulfonic acid – 99%,lauric acid – 99%, palmitic acid – 99%, ethylene glycol – 99%, ethanol – 95%, methanol – 99%, ascorbic acid – 99%, silver carbonate – 99% in these experiments.
Obtain of Silver Salts
Silver TrifluoroacetateCF3COOAg (1a)
To 5.0 g (18 mmol) of silver carbonate is added 60 ml of trifluoroacetic acid and is stirred for 30 minutes. The reaction mixture isboiled down to dryness and isreceived7.0 g (72%) of a white crystalline solid. Calculated: C – 10,874. Found C – 10,861.
Silver MethanesulphonateCH3SO3Ag(1b)
5.0 g (18 mmol) of silver carbonate is dissolved in 30 ml of methanesulfonic acid. To this solution isadded 50 ml of isopropanol, after which there is the precipitate. The reaction mixture is cooled to 0 ° C and the precipitate is filtered off, after which it is washed with 10 ml of isopropanol and winded.Obtained 5.91 g (80%) of silver methanesulfonate. Calculated: C – 5,917, H – 1.499; S – 15.798. Found: C – 5,978; H – 1.517; S – 15.826.
Silver Laurate C11H23COOAg(1c)
To 5.0 g (22.4 mmol) of the sodium saltsolution of lauric acid in 300 ml of distilled water is added dropwise a solution of 3.8 g (22.4 mmol) of silver nitrate in 170 ml of distilled water. The reaction is stirred for two hours, white precipitate. The precipitate is filtered off, washed with 100 ml of water and dried in a desiccator. Obtained 4.14 g (60%) silver laurate. Calculated: C – 46,925; H – 7,548. Found: C – 46,931; N – 7.530.
Silver methanesulphonate CH3SO3Ag (1b)
To 5.0 g (13.8 mmol) of the sodium salt suspension of palmitic acid in 300 ml of distilled water is added dropwise a solution of 2.34 g (13.8 mmol) silver nitrate in 170 ml of distilled water. The reaction is stirred for two hours, white precipitate. The precipitate is filtered off, washed with 100 ml of water and dried in a desiccator. Obtained 5.8 g (89%) of silver palmitate.Calculated: C – 52,850; H – 8,531.Found C – 52,939; H – 8,510. The received silver salt was used to modify UHMWPE.
The Polyol Synthesis
Method 1.1
To 10 ml of ethylene glycol was added 0.5 g of UHMWPE, thenit was heated to 115°C and stirred for one hour. After thatwas poured 20 micromole of the solution silver salt of 1a, c in 10 mL of ethylene glycol and kept at this temperature for 1 hour. UHMWPE is filtered off, washed with 250 ml of distilled water and dried at 110°C.
Method 1.2
To a solution of 20 micromole of silver salt 1a, c in 10 mL of ethylene glycol was added 0.5 g of UHMWPE and heated to 115°C and sonicated with frequency 20000 Hz for 30 minutes. UHMWPE was filtered off, washed with 250 ml of distilled water and dried at 110°C.
Processing of Polymer with Colloid Silver Nanoparticles
Method 2.1
To a solution of 20 micromole of silver salt 1a-e in 20 ml of solvent 2 (C = 10-3 M) solution with stirring, was poured 1.76 mg (10 micromole) of ascorbic acid in 10 milliliters of solvent 3 (C = 10-3 M). Then was added 0.5 g of UHMWPE which is pre-impregnated with ethanol and is stirred for 2 hours. The suspension is filtered on a Shott filter, washedthe precipitate with 500 ml of distilled water and dried at 110°C.
Method 2.2
To a solution of 40 micromoles of silver salt 1a-e in 20 ml of solvent 2 (C = 2 • 10-3 M) was pouredwith stirring the solution 3.52 mg (2 • 10 micromoles) of ascorbic acid in 10 milliliters of solvent 3 (C = 10-3 M). Then was added 0.5 g of UHMWPE, which is pre-impregnated with ethanol and stirred for 2 hours. The suspension is filtered on a Shottfilter, the precipitate is washed with 500 ml of distilled water and dried at 110°C.
Recovery of Silver Salt Solution in the Polymer Matrix UHMWPE
Method 3.1
To a solution of 20 micromole 1a-e of silver salt in 20 ml of solvent 2 (C = 10-3 M)was added 0.5 g of UHMWPE, which is pre-impregnated with ethanol. To the resulting suspension under stirring solutionis poured 1.76 mg (10 micromole) of ascorbic acid in 10 milliliters of solvent 3 (C = 10-3 M) and stirred for 2 hours. The suspension was filtered on a sintered glass filter, the precipitate was washed with 500 ml of distilled water and they dried at 110°C.
Method 3.2
To a solution of 40 micromoles 1a-e the silver salt in 20 ml of solvent 2 (C = 2 • 10-3 M) was added 0.5 g of UHMWPE, which is pre-impregnated with ethanol. To the resulting suspension waspoured under stirring solution 3.52 mg (10 micromole) of ascorbic acid in 10 milliliters of solvent 3 (C = 2 • 10-3 M) and stirred for 2 hours. The suspension was filtered on a Shottfilter, the precipitate was washed with 500 ml of distilled water and dried at 110°C.
Antimicrobial Activity
Antimicrobial activity of the samples was determined towards to gram-positive bacteria Staphylococcus aureus and to gram-negative bacteria Escherichia coli. Test organism E. coli was cultured on meat-peptone broth, and S. aureuson meat-peptone broth with 6% NaCl. To assess the antibacterial activity of microbial of UHMWPE powder they seed themicroorganisms cultures in the meat-peptone at a concentration from 102 to 107 CFU/ml, after thatthey add the test samples of composite powders, which activity qualitatively was assessed by the presence or absence of meat-peptone broth turbidity due to bacteria propagation. The samples were incubated at 37°C during the day.
Results and Discussions
There were consideredthree modification methods of UHMWPE with silver nanoparticles: 1) the polyol synthesis2)Processing of polymer with colloid silver nanoparticlespolymer processing silver nanoparticle colloid and 3) Recovery of silver salt solution in the polymer matrix UHMWPE.
The polyol synthesis
According to the first method the recoveringof silver salt is getting with glycol aldehyde, which is forming under the influence of a high temperature from the glycol. According to the atomic-emission spectrometry with inductively coupled plasma the silver content of UHMWPE is not more than 0.07%, according to scanning electron microscopy (SEM) there is a significant amount of aggregated silver in polymer (data shown in Table 1).
Table 1: Atomic emission spectroscopyand SEM datain the modification of UHMWPE with silver nanoparticles by polyol.
|
Method |
Silver salt |
Silver content, % |
Size distribution of nanoparticles of dispersion |
|
1.1 |
1a |
0.05 |
A significant amount of silver nanoparticles with the size of 60-110 nm was found. There is an aggregation of silver. |
|
1.2 |
0.07 |
||
|
1.1 |
1c |
0.04 |
|
|
1.2 |
0.07 |
The second method is that the UHMWPE powder which is previously wetted with ethanol,is impregnated with stirring for 2 hours by the silver colloidal solution, which is obtained previously [18, 19]. Then the powder is washed with distilled water and dried (see Table 2). Thus stable dispersion was obtained previously with the reduction of silver trifluoroacetate [18] and silver methanesulphonate [19].
Table 2: atomic emission spectroscopy and SEM data in the modification of UHMWPE with impregnation with colloidal solution of silver nanoparticles
|
Method |
Silver salt |
Solvent 2 |
Silver content, % |
Nanoparticles |
|
2.1 |
1а |
ethylene glycol |
0.22 |
65-100 |
|
2.2 |
0.35 |
60-100 |
||
|
2.1 |
1b |
0.11 |
65-90 |
|
|
2.2 |
0.30 |
70-95 |
||
|
2.1 |
1c |
0.19 |
70-110 |
|
|
2.2 |
0.33 |
70-110 |
||
|
2.1 |
1d |
methanol |
0.14 |
65-100 |
|
ethanol |
0.12 |
70-110 |
||
|
1e |
methanol |
0.04 |
70-100 |
|
|
ethanol |
0.03 |
65-95 |
According to the third method, to a solution of a silver salt in an organic solvent they add UHMWPE powder, which is impregnated with ethanol previously, and then they add a reducing agent (table 3). It should be noted that pre-impregnation of ultra-high molecular polymer powder with ethanol ensures quick absorption of solution in volume of the polymer matrix.
Figure 1 shows the receivedwith the SEM method,the image of UHMWPE powder, which is modified with silver nanoparticles. With using trifluoroacetate, methanesulfonate, laurate, silver nitrate there was found a significant amount of silver nanoparticles with the size of 60-110 nm equally spaced in the UHMWPE matrix. In the samples obtained by treating of UHMWPE powderwith a dispersion of nanosilver is observed nonequilibrium distribution of silver nanoparticles by using the fatty acid there is the presence of aggregating. With recovering silver palmitate in both cases there is an aggregation of silver, the colloidal solution is poor, and therefore there is only a small amount of nanoparticles on the UHMWPE matrix.
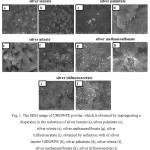 |
Figure 1: The SEM image of UHMWPE powder, which is obtained by impregnating a dispersion in the reduction of silver laurate (a), silver palmitate (c), silver nitrate (e), silver methanesulfonate (g), silver trifluoroacetate (i); obtained by reduction with of silver laurate UHMWPE (b), silver palmitate (d), silver nitrate (f), silver methanesulfonate (h), silver trifluoroacetate (j) |
Table 3: Atomic emission spectroscopy and SEM data in the formation of silver nanoparticles in a polymer matrix UHMWPE
|
Method |
Silver salt |
Solvent2 |
Silver content, % |
Nanoparticles size, nm |
|
3.1 |
1а |
ethylene glycol |
0.27 |
65-100 |
|
3.2 |
0.44 |
60-100 |
||
|
3.1 |
1b |
0.15 |
65-90 |
|
|
3.2 |
0.35 |
70-95 |
||
|
3.1 |
1c |
0.24 |
70-110 |
|
|
3.2 |
0.37 |
70-110 |
||
|
3.1 |
1d |
methanol |
0.26 |
65-100 |
|
ethanol |
0.22 |
70-110 |
||
|
1e |
methanol |
0.07 |
70-100 |
|
|
ethanol |
0.06 |
65-95 |
The content of silver nanoparticles in the polymer ranges by using different salts, salt concentration, the reducing agent and the reaction conditions. According to the atomic-emission spectrometry with inductively coupled plasma, the best results is observedwhen they use as precursors the silver trifluoroacetate – in this case, the content of silver in the polymer is 0.22 – 0.44%; silver nitrate – 0.19 – 0.37%, silver methanesulfonate 0.11 – 0.35%. In view of the low solubility of silver salts of fatty acids it is impossibleto increase the salt concentration in the organic solvent. Thus, when using silver laurate, silver content can not exceed 0.26%, silver palmitate – 0.07%.
The specific surface of nanoparticles according to the data obtained by the analyzer nanoparticle surface area is 7.1-7.6 m2/g.
To monitor the transfer completeness of passage nanosilver from the solution into the UHMWPE matrix there had been taken spectra of the filtrate absorption.Figure 2 shows that onthe absorption spectrum of the filtrate there is no peak at a wavelength of 400-420 nm, which corresponds to the peak of the plasmon resonance of silver nanoparticles, it indicates about complete or almost complete absence of silver nanoparticles in the filtrate and their sorption on the polymer matrix.
Also, using an integrating sphere there wereread spectra of thereflectance diffuse UHMWPE powders, modified with the silver nanoparticles (Fig. 3). When there is acomparison with the spectrum of the original powder UHMWPE there is minimum at a wavelength of 400 nm, which allows to judge about the presence of silver nanoparticles. Also it should be noted that this minimum corresponds to the peak of the plasmon resonance of silver nanoparticles in dispersion (Fig. 2).
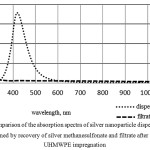 |
Figure 2: The comparison of the absorption spectra of silver nanoparticle dispersion, which is obtained by recovery of silver methanesulfonate and filtrate after UHMWPE impregnation |
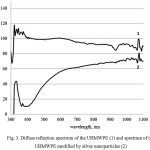 |
Figure 3: Diffuse reflection spectrum of the UHMWPE (1) and spectrum of the UHMWPE modified by silver nanoparticles (2) |
Samples, which were obtained by reduction of silver trifluoroacetate and methanesulfonate were investigated for bactericidal activity. The results are shown in the Table 4.
Table 4: The effect of UHMWPE powder on the antibacterial activity
|
Samples |
S. aureus, CFU/ml |
E. coli, CFU/ml |
|||||
|
102 |
104 |
106 |
107 |
104 |
106 |
107 |
|
|
silver methanesulfonate |
– |
± |
+ |
+ |
– |
± |
± |
|
silver trifluoroacetate |
– |
± |
+ |
+ |
– |
± |
± |
|
control |
+ |
+ |
+ |
+ |
– |
+ |
+ |
Table 4 shows that UHMWPE powder containing silver nanoparticles in a varying degree have a antimicrobial activity.The fullgrowth inhibition of S. aureuswith the samples obtained by reduction of silver trifluoroacetate and methanesulfonateis at an initial concentration of 102 CFU/mlbut at the initial concentration of 104 CFU/ml there is a partial suppression of bacterial growth.
The growth of E. coli in the control is observed at an initial concentration of 106CFU/ml. At the initial concentration the both samples completely inhibit bacterial growth, and at a concentration of 107 CFU/ml – in part.
Conclusion
There were considered approaches to modification of ultrahigh molecular weight polyethylene with silver nanoparticles. It was found, thatfor the best way ofmodificationof UHMWPE with silver nanoparticles, the most suitable are precursors such as trifluoroacetate, methanesulfonate, nitrate and silver, and the recovery of silver salt solution should be carried out in the matrix of UHMWPE. The obtained samples have antibacterial activity towards toE. Coli and S. Aureus.
Acknowledgments
The work was supported by the Russian Federation Ministry of Education and Science under the grant agreement № 14.576.21.0024 of 27 June 2014 (the project № RFMEFI57614X0024).
References
- GalibeevS.S.; KhayrullinR.Z.; ArhireevV.P. UHMWpolyethylene. Trends and Prospects.J. Bulletin of Kazan Technological University.2008, 2, 50-55.
- Domenech B.; Munoz M.; Muraviev D.N.; MacanasJ. Polymer-Silver Nanocomposites as Antibacterial materials. Formatex.2013, 630-640.
- RevinaA.A.; EgorovaE.M. Possibilities of application of nanotechnology in the production of paints and coatings. Chemical industry.2001, 4.28-32.
- Patent RU 02437645 C1, 27.12.2011. Kalivradzhiyan E.S.; Kalinichenko V.S.; Kalinichenko N.V.; Kalinichenko T.P.; Lakiza V.V.; Podoprigora A.V.; Pozov D.T.; Removable plate dentures with silver in nanoform.
- Litmanovich O.E. Patterns of interaction of macromolecules with the nanoparticles of metals and synthesis psevdomatrichny sols of metal-polymer nanocomposites. Macromolecular compounds. 2008, 7, 1370.
- Bedanokov A.Y.; Borisov V.A.; Mikitaev A.K.The properties of polymer nanocomposites.Plastics.2007, 5, 26.
- Sokolov V.I. Chiral stereochemistry nanoparticles.Coordination chemistry.2009, 8,563-575.
- Nyquist N.E.; Lysenko E.L.; Serimaa R.; Pirkkalainen K.; Vainio U.; Lavrent’ev V.K.; Medvedev D.A.; Shahmin A.L.; Saprykin N.N.; Novoselov N.P. Cellulose as a nanoreactor for nickel nanoparticles.Macromolecular compounds.2008, 1, 63 –70.
- Vishnjakova E.A.; Zhukov S.V.; Markov S.M.; Likhatskaya M.N.; Mikhalin Y.L. Determination conditions for the formation of silver nanoparticles in the reduction of glucose in aqueous solutions.Journal of Siberian university. Chemistry.2009, 2, 48-55.
- Kreibig U.; Vollmer M.; Optical Properties of Metal Clusters.The Springer Series in Materials Science. Berlin: Springer.1995, 234.
CrossRef - Abdullin S.N.;Stepanov A.L.;OsinYu.N.;Khaibullin I.B. Kinetics of silver nanoparticle formation in a viscous-flow polymer. Surf. Sci.1998,395, 242–245.
CrossRef - Quinten M.;Heilmann A.;Kiesow A. Refined interpretation of optical extinction spectra of nanoparticles in plasma polymer films. Appl. Phys. 1999,4, 707–712.
CrossRef - VishnyakovE.A.; SeljutinG.E.; GavrilovY.Y.; SaykovaS.V.; RomanchenkoA.S.; MazurovE.V.; A.N.; KokorinA.N.; MikhlinY.L.Preparation of composites based on ultrahigh molecular weight polyethylene having antibacterial properties. Journal of Siberian Federal University.Chemistry.2013,4, 372–379.
- Wiley B.; Sun Y.;Mayers B.; Xia Y. Shape-controlled synthesis of metal nanostructures: the case of silver. Chem. Eur. J..2005,2, 454–463.
CrossRef - Sun Y., Xia Y. Large-Scale Synthesis of Uniform silver nanowires through a soft self-seeding. polyol process. Adv. Mater.2002,11, 833–837.
CrossRef - Chen C.; Wang L. Yu H.; Jiang G.; Yang Q.; Zhou J.; Xiang W.; Zhang J. Study on the growth mechanism of silver nanorods in the nanowire-seeding polyol process. Mater. Chem. Phys. 2008,107, 13–17.
CrossRef - Silvert P.-Y., Herrera-Urbina R. Preparation of colloidal silver dispersions by the polyol process Part 1 -Synthesis and characterization.J. Mater. Chem.1996,4, 573–577.
CrossRef - Glushko V.N., Sadovskaya N.Y., Usova O.A., Blokhina L.I., Kozhukhov V.I.Features of Obtaining Silver Nanoparticles in Non-Aqueos Media by Reduction of Silver Trifluoroacetate. Orient J Chem.2015,4, 2515-2520.
CrossRef - Glushko V., Blokhina L., Sadovskaya N., Kozhukhov V.Nonaqueous preparation of stable silver nano particles dispersions from organic sulfonic acids. Orient J Chem. 2016,2, 789-797.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









