Partial Characterization of Chitosanase from Digestive Tract of Channastriata
Ace Baehaki1*, Shanti Dwita Lestari1, Nuni Gofar2 and Yudi Wahidman1
1Department of Fisheries Product Technology, Faculty of Agriculture, SriwijayaUniversity, Indralaya, South Sumatera, Indonesia.
2Department of Soil Science, Faculty of Agriculture, SriwijayaUniversity, Indralaya, South Sumatera, Indonesia.
Corresponding Author E-mail: ace76_none@yahoo.com
DOI : http://dx.doi.org/10.13005/ojc/320647
The purpose of this study wasto partially characterize chitosanase isolated from snakehead fish’s (Channa striata) digestive tract.Two types of snakehead fish, domesticated and non-domesticated, were used as samples in order to assess the effect of feeding types on chitosanase activity. Chitosanase isolated from the stomach andintestine of domesticated snakehead fish had higher activity than non-domesticated samples with the value of 0.0395 U/mL and 0.0296 U/mL, respectively. The optimum chitosanase activity from the stomach and the intestine of domesticated fish was at pH 7. Lower optimum pH (6) was noticed for non domesticated samples. Chitosanase from both samples showed optimum activity at 70 °C.
KEYWORDS:characterization; chitosanase; digestive tract; snakehead
Download this article as:| Copy the following to cite this article: Baehaki A, Lestari S. D, Gofar N, Wahidman Y. Partial Characterization of Chitosanase from Digestive Tract of Channastriata. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Baehaki A, Lestari S. D, Gofar N, Wahidman Y. Partial Characterization of Chitosanase from Digestive Tract of Channastriata. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=25322 |
Introduction
Chitin is the second largest polysaccharide after cellulose which has the chemical formula of poly (2-acetamide-2-dioksi-β-D-Glucose) with β- glycosidic (1.4) linkage connecting each unit. The chemical structure of chitin is similar to cellulose, only differentiated by a group attached to the atom C2. If the cellulose cluster bound to the atom C2 is OH, then the chitin-bound is acetamide group1. Chitosan is a deacetylated product of chitin which is found in many insects, crustaceans and fungi2. Chitin is a fibrous material and very insoluble, while chitosan is slightly soluble in water and is much more tractable material with a broad range of applications3.
Chitosan is also found in nature; found in the insect cuticle4 and the cell walls of fungi class Zygomycetes, in chlorophycean algae Chlorella sp.5. This chitosan naturally synthesized by the joint action of chitin synthetase and chitin deacetylase, as shown for Mucorrouxii and Colletotrichum lindemuthianum6.
Materials and Methods
Chitosanase Activity
Chitosanase assay was conducted according to Yoon et al.7, with modification. One (1) unit of chitosanase activity was defined as the amount of the enzyme which produces 1 μmol of reducing sugar (glucosamine) per minute.
Effcet of pH Enzyme Activity
Optimum pH was determined by assaying in buffer with pHvalues of 6-9 using citric acid buffer (pH 3-4), citric-phosphate buffer (pH 4-6), phosphate buffer (pH 6-8) and boric buffer (pH 8-9), in thepresence of soluble chitosan substrate.
Effcet of Temperature on Enzyme Activity
Optimum temperature was measured at 30, 40, 50, 60, 70 and 80 °C at pH 7 by using soluble chitosan as the substrate.
Result and Discussion
Chitosanase Activity
The results of the chitosanase activity fromdigestive tract of Snakehead fish (Channastriata) are presented in Figure 1.
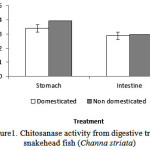 |
Figure 1: Chitosanase activity from digestive tract of snakehead fish (Channastriata) |
Fig1 shows two different chitosanase activity of Channastriata (domesticated and non domesticated fish). Enzymes isolated from both stomach and intestine of domesticated fish had higher activity in the amount of 0.0395 U/mL and 0.0296 U/mL respectively.Chitosanaseisolated from the stomach of domesticated and non domesticated samplesconsiderably showed higher activity than the same enzyme isolated from the intestine.
Effect of pH on Chitosanase Activity
Effects of pH on the activity of chitosanase isolated from digestive tract of Channastriataare presented in Figure 2 and 3.
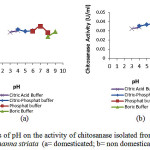 |
Figure 2: Effects of pH on the activity of chitosanase isolated from digestive tract of Channastriata (a= domesticated; b= non domesticated) |
Optimumchitosana sea ctivities from the stomach and the intestine were at pH 7 (domesticated) and pH 6 (non domesticated).The results were higher than pH 5.8 of Bacillus cereus8, pH 5.0 of Streptomyces cyaneogriseus9and pH 6 of Paenibacillus ehimensis10.
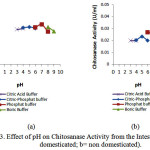 |
Figure 3: Effect of pH on Chitosanase Activity from the Intestine (a= domesticated; b= non domesticated). |
Effect of Temperature on Chitosanase Activity
Temperature ranged between 30 oC and 80 oC were used to study the effect of pH on chitosanase activity. Fig 4 showed the effect of temperature on the activity of chitosanaseisolated from digestive tract of Channastriata.
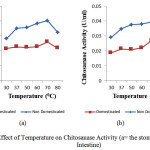 |
Figure 4: Effect of Temperature on Chitosanase Activity (a= the stomach; b=the Intestine) |
The optimum temperature of 70°C was recorded for the chitosanase(the stomach and the intestine) in this study. The result was higher than the 54°C optimum temperature reported for Bacillus cereus8, 50 oCforBacillus megaterium11, and Gongronella sp.12.
References
- Muzzarelli, R.A.A, Chitin. In: AspinallGO (ed), The polysaccharides, Academic Press London, UK, 1985, 3,417-447.
- Sanford PA and Hutchings GP, Chitosan-A natural, cationic biopolymer: commercial application in Industrial polysaccharides, Amsterdam, Elsevier,1987, 365-371
- Hirano S, Chitin and Chitosan.Production and Application of Chitin and Chitosan in Japan. Essex Elsevier, 1989, 37–43.
- Aruchami M, Gowri N and Sundara-Rajulu G, Chitin deacetylases in invertebrates in Muzzarelli RAA, Jeuniaux C and Gooday GW (ed), Chitin on nature and technology, Plenum Press, New York,1986
- Mihara S, 1961, Change in glucosamine content of chorella cells during the course of their life cycle, Plant Cell Physiol, 1961, 2, 25-29.
CrossRef - Davis LL and Bartnicki-Garcia S, The coordination of chitin and chitosan synthesis in Mucorrouxii, J Gen. Microbiol, 1984, 130, 2095-2102.
- Yoon HG, Kim HY, Kim HK, Shin DH, Jng KC and Park RD, ProtExpr. Purif, 2006, 45, 125
- Piza FAT, Siloto AP, Carisalho GV and Franco TT, Purification and characterization of chitosanase from Bacillus cereus, BrazzJ ChemEng, 1999, 16, 185-192.
CrossRef - El-Sherbiny ESA, Purification and characterization of chitosanase enzyme from Streptomyces cyaneogriseus, Asian J BiolSci, 2011, 4, 15-24.
- Pagnoncelli MGB, de Araujo NK, da Silva NMP, de Asis CF, Rodrigues S and de Macedo GR, Chitosanase production by Paenibacillusehimensis and its application for chitosan hydrolysis, Brazz Arch BiolTechnol, 2010, 53, 1461-1468.
CrossRef - Pelletier A and J. Sygusch, Purification and characterization of threechitosanase activities from Bacillus megateriumP1, Appl. Environ. Microbiol, 1992, 56, 844–848.
- Zhou W, Yuan H, Wang J and Yao J, Production, purification and characterization of chitosanase produced by Gongronella sp. JG. LettApplMicrobiol, 2008, 46, 49–54.

This work is licensed under a Creative Commons Attribution 4.0 International License.









