Kinetic Modeling of Photocatalytic Degradation of Alachlor using Tio2 (Degussa P25) in Aqueous Solution
Thanikachallam Pushpa Malini1*, Atmakuru Ramesh2, Johnpeter Arockia Selvi1, Maruthapillai Arthanareeswari1 and Palanisami Kamaraj1
1Department of Chemistry, SRM University, Kattankulathur – 603 203, Tamil Nadu, India.
2Department of Chemistry, International Institute of Biotechnology and Toxicology (IIBAT), Padappai, Chennai - 601 301, Tamil Nadu, India.
Corresponding Author E-mail: pushpamalini24@gmail.com
DOI : http://dx.doi.org/10.13005/ojc/320639
A kinetic model was developed to simulate the solar photocatalytic degradation of alachlor (organo chlorine herbicide) in aqueous solution using TiO2 (Degussa P25) catalyst. The effect of temperature, pH, radiant flux, TiO2 loading, presence of various cations, anions, surfactants on the photocatalytic degradation of alachlor were studied and determined the optimum condition under which the degradation rate was maximum. The solar irradiated samples were analyzed by a validated HPLC method. The photoproducts were determined by using LC/MS/MS technique. Rapid dissipation of herbicide was observed by using 0.5g/l of TiO2 (Degussa P-25) catalyst. The rate of degradation was found to increase with the increase in the temperature from 40°C to 70°C. The influence of cations on the degradation rate was in the order Fe2+, Cu2+ >Zn2+>Ni2+ >Mn2+ > Co2+ and for anions Cl-, HClo3- > CO32-, So42-. The rate was decreased as the pH was increased from 4.0 to 8.0. But at very high alkaline condition (pH 10.0) the rate was found to be the maximum. Among the three surfactants used (Cetyltrimethylammonium bromide (CTAB), Sodium dodecyl sulphate (SDS) and Polyethylene (23) dodecanol (Brij-35)) Sodium dodecyl sulphate showed much influence and when the study was conducted at three different radiant flux (109 Lux, 655 x 101 Lux, 601 x 102 to 1074 x 102Lux) it was observed that the rate was faster under the day light flux 601 x 102 to 1074 x 102Lux. The degradation rate of alachlor was found to follow the first order kinetics. Finally the optimized conditions or the kinetic model which showed maximum degradation rate was then applied for treating effluent and ground water.
KEYWORDS:Alachlor; TiO2 (Degussa P-25); Photocatalytic degradation; Kinetic modeling
Download this article as:| Copy the following to cite this article: Malini T. P, Ramesh A, Selvi J. A, Arthanareeswari M, Kamaraj P. Kinetic Modeling of Photocatalytic Degradation of Alachlor using Tio2 (Degussa P25) in Aqueous Solution. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Malini T. P, Ramesh A, Selvi J. A, Arthanareeswari M, Kamaraj P. Kinetic Modeling of Photocatalytic Degradation of Alachlor using Tio2 (Degussa P25) in Aqueous Solution. Orient J Chem 2016;32(6). Available from: http://www.orientjchem.org/?p=25411 |
Introduction
The intensive use of pesticides has been one of the factors contributing to high crop yields and lower commodity prices. However there is increasing concern about the impact of pesticides on human health and environment. Pesticide contamination may affect the quality of soil, water, air and consequently may pose risk to flora and fauna. At present pollution of the environment by pesticides is one of the main health risk factors [1, 2]. The extent of pesticide contamination of the water environment has recently raised much concern because of the potential health hazards associated with the entry of these compounds into the food chain of human and animals. [3, 4]. Hence an effective control of maximum admissible concentrations of pesticides in water, soil, crops and food is essential for improving the quality of life.
There are three main paths for the destruction of organic compounds. First, light can directly excite the molecules of the organic contaminants thus leading to their direct photochemical destruction. Second, light can activate some exogenic oxidants like H2O2, O3 which when added in to the system produce strong oxidizers like OH∙ or O– and third, light can be adsorbed by some photocatalyst like TiO2 etc., which either directly participate in the oxidation of organic substance or initiate the formation of OH radicals from water and H2O2.
Many toxic organic compounds are designed to be chemically stable and thus resistant to conventional microbial degradation. Advanced oxidation process (AOPs) are considered to be promising in the abatement of the growing environmental problems resulted from these toxic compounds [5]. Among the semiconductor substances, TiO2 has been extensively applied in researches because of its non-toxicity, chemical stability, low cost and suitable to work under sunlight as energy source [6-10]. TiO2 powder (Degussa P-25), which is a standard material in the field of photocatalytic reactions, contains anatase and rutile phases in a ratio of about 3:1. It is produced through high temperature (> 1200°C) flame hydrolysis of TiCl4 in the presence of H2 and O2. TiO2 so formed is treated with steam to remove HCl which is also produced as a part of the reaction. The product is 99.5% pure TiO2 (anatase: rutile, 70:30) which is non-porous, with rounded edges cubic particles. The Degussa P-25 TiO2 powder has a surface area of 50 ± 15 m2g-1 and an average particle diameter of 21 nm. Transmission electron microscopy distinguished anatase and rutile particles separately form their aggromelates. Theurich et al., 1996 found that the rutile phase does not exist as an overlayer on the surface of the anatase particle but it exists separately from anatase particles [11].
The photocatalyst derives its activity from the fact that when photons of a certain wavelength are incident upon its surface, electrons are promoted from the valence band and transferred to the conductance band. This leaves positive holes in the valence band, which reacts with the hydroxylated surface to produce OH· radicals, which are the most potent oxidizing agents.
In the absence of suitable electron and hole scavengers, the stored energy is dissipated within a few nanoseconds by recombination. If a suitable scavenger or a surface defect state is available to trap the electron or hole, their recombination is prevented and subsequent redox reaction may occur. In Degussa P-25 the conduction band electron of the anatase phase jumps to the less positive rutile phase, reducing the rate of combination of electrons and holes in the anatase phase.
Organochlorine pesticides have given rise to the greatest environmental concern because of their greater stability in the aquatic ecosystem. In general, they are resistant to hydrolysis, and those that undergo photochemical reaction (usually by photosensitizing) tend to form compounds with persistence comparable to, or greater than, their parent compounds [12,13]. They are also insoluble in water and the environmental contents have focused mostly on their potential for biological accumulation.
Alachlor chemically known as 2-chloro-2′,6′-diethyl-N-methoxymethyl acetanilide is a pre-emergence herbicide mainly used to control annual grasses and many broad-leaved weeds in cotton, brassicas, maize, oil seed rape, peanuts, radish, soya beans and sugar cane.
This paper reports the behavior of alachlor degradation in water and the influence of different physico – chemical parameters on the rate of photocatalytic degradation of alachlor in aqueous solutions using the photocatalyst TiO2 (Degussa P-25).
Materials and methods
Alachlor (purity 99%) is obtained from Chem Service, West Chester, England. Acetonitrile used for the liquid chromatography was of residue grade (E.Merck, Germany). All the photocatalytic degradation assays were carried out using titanium dioxide (Degussa P-25) (Sigma Aldrich, Germany), which is predominantly anatase. It is nonporous and offers a specific surface area of about 50 m2g-1 and a density of 3.85gcm-3.
Alachlor samples were analysed by Shimadzu High Performance Liquid Chromatograph system equipped with LC-10 AT pump and SPD-10A UV-VIS detector connected to CBM-101 module using CLASS-LC10 software. The column used was Phenomenex C18 (25 cm length, 4.6 mm internal diameter). Mobile phase was Acetonitrile: Water (80: 20). The analysis was performed at the Wavelength 220 nm in the flow rate 1.0 ml/min. Approximate retention time for alachlor was 8.2 minutes.
From the stock solution (500mg in 100 ml of residue grade acetonitrile) of alachlor, desired concentrations of working solutions were prepared by serial dilution. Two control samples were maintained for this purpose. One without the addition of TiO2, and the other without the addition of alachlor. 0.5g/l TiO2 (amount was fixed after studying the influence of catalyst loading on herbicide degradation) was added to the working solution and all the samples were exposed under the direct sunlight during the daytime. The light intensity during the day time was observed as 601 x 102 to 1074 x 102 lux. Aliquots of sample solution were collected and centrifuged at 3000 rpm. The filtrate was again filtered through 0.4µm nylon (Millipore) filter paper to separate the tiny particles of the catalyst (TiO2). The clear samples were then analysed by validated HPLC.
Effect of TiO2 amount on photodegradation
Five sets of 20 µg/ml of alachlor solution were made and to each of the solution different known amounts of TiO2, 10mg, 20mg, 50mg 100mg and 150mg were added. The samples were exposed to the direct sunlight while stirring continuously. The amount of TiO2 at which maximum herbicide degradation occurred was identified and used in the subsequent studies.
Effect of temperature on photodegradation
To a 20µg/ml of alachlor solution, 0.5 g/l of TiO2 was added. The samples were then placed inside a thermostatically temperature controlled water bath at a specified temperature. The entire system was placed under the sunlight for solar irradiation. The study was conducted at four different temperatures 40, 50, 60 and 70 ± 1°C. At different time points samples were collected, centrifuged and filtered through 0.4µm nylon (Millipore) filter paper for complete separation of TiO2 particles.
Effect of herbicide concentration on photodegradation
The dissipation of alachlor at five different concentrations were studied using optimum amount of TiO2 (0.5g/l) at the optimum temperature 40 ± 1°C. The concentrations of herbicides studied were 10, 20, 50, 100 and 500µg/ml.
Influence of cations and anions
To 20µg/ml concentration of alachlor solution added 0.1 ml of metal/anion stock solution, to give different uniform concentrations of ionic solution (10-2M, 10-3M and 10-4M). 0.5g/l of TiO2 was then added. After mixing the solutions vigorously, the compounds were kept in water bath at constant temperature 40±1°C and exposed to sunlight while stirring the contents constantly. The metal ions used in the studies were Ferrous sulphate (Fe 2+), Copper sulphate (Cu2+), Cobalt nitrate (Co2+), Nickel sulphate (Ni2+), Manganous sulphate (Mn2+) and Zinc sulphate (Zn2+). The anions used in the study were Sodium sulphate (SO42-), Sodium chloride (Cl–), Sodiun perchlorate (ClO4–), Sodium carbonate (CO32-), Sodium bicarbonate (HCO3–).
Effect of pH on photodegradation
Four different buffer solutions of pH 4.0, 6.0, 7.0 and 8.0 were prepared to evaluate the effect of pH on the rate of hydrolysis. Known quantity of a herbicide was added to each of the buffer solution to obtain the final concentration 20 µg/ml. To this herbicide spiked buffer solution 0.5 g/l of TiO2 was added. Studies were conducted at 40°C
Effect of surfactants on photodegradation
To a known concentration of alachlor solution, spiked 0.1 ml stock solution of a surfactant to give a concentrations of 10-3M. 0.5g/l of TiO2 was then added. After mixing the solutions vigorously, the compounds were kept in water bath at 40°C and exposed to sunlight while stirring the contents constantly. The surfactants used in the study were cationic surfactant cetyltrimethylammonium bromide (CTAB), anionic surfactant sodium dodecyl sulphate (SDS), and non-ionic surfactant polyethylene (23) dodecanol (Brij-35).
Effect of radiant flux on photodegradation
To a 20µg/ml of herbicide solution, 0.5 g/l of TiO2 was added. The samples were then placed inside a water bath at a specified temperature. Radiant flux was observed at room condition using Lux meter which was capable of measuring radiant flux up to 50,000 Lux. Dissipation of herbicide was monitored at different time points in room light. Simultaneously two sets of solutions of 20µg/ml of herbicide concentration containing 0.5g/l of TiO2 were prepared. One of which was kept under day light and another under mercury lamp of 300 watts.
During each study samples were collected at different time points, centrifuged and filtered through 0.4 µm nylon (Millipore) filter paper for complete separation of TiO2 particles. The filtrate was then analysed by HPLC
Identification of metabolites
The importance of degradation products must be stressed in the case of active compounds which may yield substances that are most active, persistent or toxic than the parent compound [14]. To identify the metabolites, if any formed during the degradation process and to asses the completion of photocatalytic degradation in aqueous solutions, fraction of solar irradiated samples collected were analysed by LC/MS/MS .
In LC/MS/MS, samples were analysed in both positive ion and negative ion mode. PE 200 quaternary pump was used under the pressure range 0 to 6100.0 Psi. PE 200 auto sampler was used. Water / Acetic acid (0.1%) / Acetonitrile were used as mobile phase for the analysis.
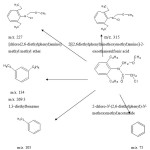 |
Scheme 1: Identified metabolites of alachlor |
Results and Discussion
Investigations were carried out to establish the photocatalytic effect on the degradation of alachlor using TiO2 as catalyst. The influence of amount of TiO2, temperature, anionic and cationic reagents, pH, surfactants and radiant flux were studied and determined the optimum condition for greater alachlor dissipation. The data generated clearly showed that the addition of TiO2 in terms of different quantities highly influenced the degradation rate. But with very high amounts of TiO2 (>500mg/L) detrimental effect was observed due to the poor light penetration in to the sample because of heavy TiO2 loading. This was evident from the fact that 0.5 g/l of TiO2 showed optimum dissipation up to the level 20µg/ml. The increase in temperature significantly increased the dissipation rate in the presence of TiO2 catalyst up to 70°C. At different concentrations of the herbicides investigated, the results showed that the dissipation was rapid up to the level 50µg/ml. At very high concentration levels (100 and 500µg/ml) the rate was slowed down. At higher concentration levels the results showed the requirement of additional quantities of TiO2 as evidenced from the presence of residues.
Anionic and cationic substances also influenced the dissipation of herbicides. The presence of anions such as chloride, perchlorate, sulphate, carbonate and bicarbonate affect the adsorption of the degrading species and thereby affecting the degradation rate. The presence of cations showed both positive and negative effect on the degradation rate. The presence of transition metal ions such as Fe2+ and Cu 2+ showed marked enhancing effect on the degradation rate. It was also observed that the degradation rate was increased with the increase in the concentration of ferric ion. The presence of the cations cobalt and manganese showed detrimental effect that may be due to the associated anion and the effect of the salts on substrate adsorption.
From the investigations it was observed that at pH 4.0, dissipation rate was enhanced and showed detrimental effect in the rate towards neutral pH. This may be due to the influence of pH on the surface charge of the photocatalyst and also the state of ionization of the substrate and hence its adsorption. At pH 4.0 and 9.0, the photocatalytic degradation was found to be higher. The faster degradation at higher pH’s can be attributed due to the rapid hydrolysis of molecules by the free OH radicals.
Effect of surfactant was studied by using cetyltrimethyl ammonium bromide (CTAB), sodium dodecyl sulphate (SDS) and polyoxy ethylene (23) dodecanol(Brij-35). The degradation of the herbicide was enhanced while using SDS surfactant. The enhancement may be due to the hydrophobic interactions of the herbicide with the anionic micelles. The inhibition of oxidation was observed while using CTAB and Brij-35 surfactants, the inhibitory effect may be due to the lack of hydrophobic or hydrophilic interactions between the herbicide and the surfactants.
The degradation rate of herbicide was found to be proportional to the radiant flux used. At higher flux the rate was greater, this means that the process works in a good photocatalytic regime, the incident photons were efficiently converted into active species that act in the degradation mechanism. The conditions under which the degradation rate of Alachlor was greatly accelerated were given in the table-1. The degradation pattern of alachlor under the optimum conditions was represented graphically in Fig.1.
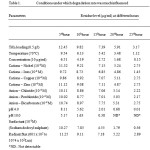 |
Table 1: Conditions under which degradation rate was much influenced |
 |
Figure 1: Graph representing the dissipation of alachlor under optimized conditions |
The applicability of the method was studied using effluent samples collected from the herbicide manufacturing facility. To understand the influence of inherent parameters associated with the effluents, effluent samples spiked with the herbicides were also tested without the addition of TiO2. Under the identical study period, 18 to 25% dissipation was observed in the samples fortified with 20µg/ml of herbicide. An identical experiment conducted in ground water clearly shows the complete dissipation of alachlor in 40 hours. At this time points no residues were detected by the HPLC technique used. This unequivocally highlights the influence of TiO2 in photocatalytic degradation of herbicide in ground water and in effluent treatment. The details were graphically represented in Fig.2.
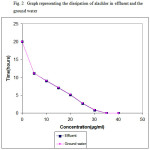 |
Figure 2: Graph representing the dissipation of alachlor in effluent and the ground water |
This was also evidenced by the confirmatory technique LC/MS/MS applied in the study. Further studies using LC/MS/MS identification of metabolites, showed the formation of several metabolites in aqueous solutions due to the photocatalytic degradation of alachlor. The details were presented in scheme 1.
The rate of the reaction was calculated under the following conditions. Concentration of herbicide – 20µg/ml, amount of TiO2 – 0.5g/l, temperature – 40°C. Calculated the dissipation kinetics in terms of DT50, DT90 and rate constant. The DT50, DT90 and rate constant of alachlor were found to be 7.68 ± 1.41, 29.42 ± 5.69 and 0.1147 hour-1respectively. Results clearly shows that the dissipation follows first order kinetics in all the investigations conducted with the alachlor.
Conclusion
The ensemble of these results clearly confirms that photocatalysis is a convenient and cheap means of decontaminating ground water and for treating effluent. The results also showed the rate of decontamination of ground water increased in several folds under various conditions established. Because of the robust character of titania, photocatalysis can be envisaged as an advantageous technique for purifying aqueous wastes [15] and producing drinking water, especially in remote arid sunny places, where water supply will be a crucial problem in the next millennium.
Acknowledgement
The authors thank International Institute of Biotechnology and Toxicology, Padappai and SRM University, Kattankulathur for providing the facilities required to complete the research work.
References
- Lu JL, Risk factors to pesticide exposure and associated health symptoms among cut-flower farmers, International J Environ. Health Res. 2005, 3, 161-169.
CrossRef - Johnson.B.L; A review of health – based comparative risk assessments in the United States, Rev. Environ. Health, 2000, 15, 273-287.
CrossRef - Piyush Gupta; Surendra Roy; Amit B. Mahindrakar, Treatment of groundwater using phytoremediation technique at Kolar Gold Fields, India, International Journal of Environmental Engineering, 2015 , 7, 11 – 34
CrossRef - Farzana Haque; Elena Vaisman; Cooper H.; Langford and Apostolos Kantza, Preparation and performance of integrated photocatalyst adsorbent (IPCA) employed to degrade model organic compounds in synthetic wastewater, Journal of Photochemistry and Photobiology A: Chemistry, 2005, 169, 1, 21-27.
CrossRef - Bessekhuad.Y.; Robert. D and Weber .J.V., Bi2S3/ TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant, Journal of Photochemistry and Photobiology A: Chemistry, 2004, 163, 569-580.
CrossRef - Ioannis K. Konstantinou and Triantafyllos A. Albanis, Photocatalytic transformation of pesticides in aqueous titanium dioxide suspensions using artificial and solar light: intermediates and degradation pathways, Applied Catalysis B: Environmental, 2003, 42, 4, 319-335.
CrossRef - Salah Rafqah, Pascal Wong-Wah-Chung, Sylvie Nelieu, Jacques Einhorn and Mohamed Sarakha, Phototransformation of triclosan in the presence of TiO2 in aqueous suspension: Mechanistic approach, Applied Catalysis B: Environmental, 2006, 66, 1-2, 119-125.
CrossRef - Masahiro Katoh, Hironori Aihara, Toshihide Horikawa and Tahei Tomida Spectroscopic study for photocatalytic decomposition of organic compounds on titanium dioxide containing sulfur under visible light irradiation, Journal of Colloid and Interface Science, 2006, 298, 2, 805-809.
CrossRef - Caimei Fan; Peng Xue and Yanping Sun, Preparation of Nano-TiO2 Doped with Cerium and Its Photocatalytic Activity, Journal of Rare Earths, 2006, 24, 3, 309-313.
CrossRef - Police Anil Kumar Reddy; Pulagurla Venkata Laxma Reddy; Vutukuri Maitrey Sharma; Basavaraju Srinivas; Valluri Durga Kumari; Machiraju Subrahmanyam, Photocatalytic degradation of Isoproturon Pesticide on C,N and S doped TiO2, J.Water Resource and Protection, 2010, 2, 235-244.
CrossRef - Theurich J; Linder M. and Bahnemann D.W. Photocatalytic degradation of 4-chlorphenol in aerated aqueous titanium dioxide suspensions, a kinetic and mechanistic study, Langmuir, 1996, 12, 6368-6376.
CrossRef - Moza. P.N.; Hustert. K.; Pal. S and Sukul.P, Photocatalytic decomposition of pendimethalin and alachlor, Chemosphere, 1992, 25, 11, 1675-1682.
CrossRef - Baker. E.J.; Adams. R.J.; Macomber. S. A.; Immunoassay screens for alachlor in rural wells; false positives and an alachlor soil metabolite, Environ. Sci. Technol. 1993, 27, 560-562.
CrossRef - Lima A and Vega L, Methyl-parathion and organophosphorous pesticide metabolites modify the activation status and interleukin-2 secretion of human peripheral blood mononuclear cells, Toxicol Lett. 2005, 158(1), 30 – 38.
CrossRef - Umar Ibrahim Gaya and Abdul halim Abdullah, Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems, Journal of Photochemistry and Photobiology C: PhotoChemistry Reviews, 2008, 9, 1 – 12.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.









