Heat-Resistant Composite Materials Based on Polyimide Matrix
Vitaly Sergeyevich Ivanov, Alyona Igorevna Wozniak and Anton Sergeyevich Yegorov*
Federal State Unitary Enterprise «State Scientific Research Institute of Chemical Reagents and High Purity Chemical Substances» (FSUE «IREA») 107076, Russia, Moscow, Bogorodskyval.
Corresponding Author E-mail: egorov@irea.org.ru
DOI : http://dx.doi.org/10.13005/ojc/320638
Heat-resistant composite materials with a polyimide-based binder were obtained in this paper. Composites were prepared with different content of single-wall carbon nanotubes (SWCNT) and nanostructured silicon carbide, and polyimides coated carbon fibers woven into the cloth. Composite materials showed high values of thermostability and resistance to thermo-oxidative degradation, as well as good mechanical properties.
KEYWORDS:Heat-resistant composite materials; carbon fiber; silicon carbide; polyimide matrix; carbon nanotube
Download this article as:| Copy the following to cite this article: Ivanov V. S, Wozniak A. I, Yegorov A. S. Heat-Resistant Composite Materials Based on Polyimide Matrix. Orient J Chem 2016;32(6). |
| Copy the following to cite this URL: Ivanov V. S, Wozniak A. I, Yegorov A. S. Heat-Resistant Composite Materials Based on Polyimide Matrix. Orient J Chem 2016;32(6).Available from: http://www.orientjchem.org/?p=25266 |
Introduction
Due to the increasing demand for structural materials, the most widely used in current technology (primarily aerospace), new chemical structures having a certain range of physical properties are constantly developing. The extensive use of composite materials (CM), especially polymeric composite materials (PCM), has resulted in the enormous progress in polymer chemistry and physics. Currently the most popular fillers among PCM are continuous filaments and textile forms as filaments, ropes, rovings, ribbons (PCM with continuous filaments, reinforced plastics). Such fillers as carbide fibers, carbon nanotubes (CNTs), fullerenes and other nano-objects have a normal rate of growth in technologies. Polymer nanocomposites that represent a new class of materials have unique barrier properties, electrical conductivity, thermal conductivity, high strength, heat resistance, thermostability and low flammability. [1-9].
Polyimides (PI) represent one of the most important polymers, commercially available for a variety of applications, such as aerospace and electronics industry, in which structural materials are often exposed to extreme conditions of mechanical, electrical, radiation, and other stresses. Despite the outstanding performance parameters of PI, these polymers have disadvantages. Among others, one of the most important disadvantage is the low bending and breaking strength and frangibility, caused by the presence of group-linkers in diamine or dianhydride fragments in the polyimide chain structure, the introduction of which is necessary to reduce the glass-transition temperature (Tg) and thus improve the processability of the polymer product [ 10-13].
Currently PIs are used as matrices to create reinforced composites based on light carbon fibers as a replacement for metal parts in the aerospace industry and airframe parts, due to their outstanding thermal and mechanical resistance. PIs are used in the cable industry in the preparation of electrical-insulating varnishes and enamels having high thermostability and elasticity, good dielectric properties for coating wires and products [14].
One of the ways to increase the mechanical parameters of PIs, is the introduction of such fillers as nanotubes, fullerenes and fibers into the polymeric structure that act as a “molecular lubricant” and allow the polymer chains move more freely relative to each other, which in turn, leads to improvement of the shear moduli, relative deformations , strength characteristics and other physical and mechanical properties [15]. Adding only 0.1% of weight of the filler, in certain cases, increases the mechanical strength considerably.
Using the light elements (like carbon, silicon or boron) is the most promising for the production of materials with high mechanical properties as the theoretical strength of the material depends on the radius of the atom, forming a chemical bond. Thermostability of PCM when filling with high-strength and stable objects (carbide fibers, nanotubes, fullerenes) [16-19] will be determined mainly by the choice of the polymer matrix, the less heat-stable component of PCM. Mechanical properties of PCM are largely determined by the properties of the filler. However, the properties of PCM depend on the ratio of matrices and fillers’ properties that determine the interaction of components, fracture toughness, crack resistance, solidity, practically the whole range of technological and operational properties of PCM.
The authors [23, 24] have chosen as a model two well-studied polyimides -LaRC CP2 and APB/ODPA, obtained by polymerization in situ. Filling these PIs with nanotubes in the range from 0.1 to 1.0% by volume, significantly increased the mechanical properties of polymers. Thus, when using the PI, CNT matrix of 1%, PCM modulus of elasticity based on LaRC CP2 is increased by 65% and 43% for APB / ODPA [25].
However, when preparing PCM based on PI as a matrix and nano-objects as a filler, we often have to face with such a problem as the inhomogeneity of the filler dispersion in the matrix, which leads to deterioration of the mechanical properties of the PCM. To circumvent difficulties associated with inhomogeneity of dispersion, we often resort to chemical inoculation of filler molecules to the end fragments of polymer chain or the side chain [26]. On the basis of the received data, it is possible notice that when the content of the modified CNTs in PCM amounts only 0.5% by weight, the tensile strength (σ) increased nearly by 1.8 times, and a modulus of elasticity (E) – by 1.2 times. It is worth noting that further filling of polyimide matrix with the filler results in a reduction of mechanical characteristics of the PCM samples. So, while filling of 5 wt.%, σ, and elongation (Δl) becomes lower than initial values for the unfilled PI, and E becomes only slightly greater than unfilled sample. These facts can be explained by the aggregation of CNT between each other due to Van der Waals interactions that make a significant contribution when increasing the concentration of CNTs in a polymer matrix.
The purpose of this paper is to obtain heat-resistant composites based polyimides with different content of single-wall carbon nanotubes (SWCNT) and nanostructured silicon carbide, as well as polyimides coated carbon fiber woven into the fabric. As used polyimide matrices are insoluble in organic solvents, and their softening temperature exceeds 300 ° C, it is advisable to use the method of in-situ polymerization. The thermostability in argon and in air and mechanical properties were measured for the composite obtained. Tensile stress and elongation at break were measured for composites with CNTs and SiC. Flexural strength and modulus of elasticity were measured for composites with HC.
Experimental Part
Process for obtaining composite polymeric materials with continuous filaments is divided into two stages: obtaining the half-finished product (saturated with pre-preg resin) of a given configuration and molding it to achieve high strength and toughness [2].
Materials and Reagents
Pyromellitic anhydride (PMDA), 3,3′, 4,4′- benzophenonetetracarboxylic acid dianhydride (BPDA) and 3,3′, 4,4′ – diphenyl oxide tetracarboxylic acid dianhydride (DPDA) were previously obtained by the authors [27]. Mass fraction of basic substance is not less than 95%. 4,4 ‘- oxydianiline (ODA) and N-methyl pyrrolidone were purchased from Vekton, 4 – aminophenylsulfone (PS) was purchased from Acros Organics. Single-walled carbon nanotubes (SWCNTs) were purchased from OCSiAl. Multiwall carbon nanotubes (MWCNTs) were purchased from NanoTechCenter. Carbon fiber (CF), woven into the cloth of the brand UT-900-PM was purchased from Argon. Nanostructured silicon carbide is purchased from InChem-Synthesis.
Preparation of Composite Materials PI-SWCNT
A three-necked 250 ml flask equipped with an ultrasonic disperser, a reflux condenser, a tube for injection of inert gas was filled with 90 ml of N-methylpyrrolidone, CNTs and 71.9 mmol 4,4′-oxydianiline. The reaction mass was sonicated at a frequency of 20 kHz for 15 minutes while cooling in an ice bath. Then it was stirred using a conventional mechanical stirrer. When 71.9 mmol of dianhydride was added small portions, and then solution stirred for 24 hours at room temperature. The resulting mixture was cast onto a ceramic tile to obtain films using an applicator and then it was placed in a vacuum oven. The film was dried in a vacuum oven at 80 ° C for 6 hours, then at 150 ° C, 200 ° C, 250 ° C and 300 ° C for 1 hour at each temperature and then at 350 ° C for 15 minutes.
Preparation of Composite Materials PI- Carbon Fiber
A three-necked 250 ml flask, equipped with a reflux condenser, a tube for injection of inert gas was filled with 40 ml of N-methylpyrrolidone and 22.9 mmol of diamine. Then, with stirring and cooling in argon flow, maintaining the temperature at 15 ° C it was added 22.9 mmoldianhydrideportionwise. When adding all of the dianhydride, the cooling was stopped and it was stirred at room temperature for 24 hours. Upon completion of mixing the carbon cloth UT-900- PM was impregnated with the resultant polyamic acid and left under pressure for 8 hours. Flowed out binder was removed with a spatula. The resulting pre-preg was spread on a ceramic tile and placed in a vacuum oven. The pre-preg was dried in a vacuum oven at 80 ° C for 6 hours, then at 150 ° C, 200 ° C, 250 ° C and 300 ° C for 1 hour at each temperature and then at 350 ° C for 15 minutes.
Preparation of Composite Materials PI-SiC
A three-necked 250 ml flask equipped with an ultrasonic disperser, a reflux condenser, a tube for injection of inert gas was filled with 55 ml of N-methylpyrrolidone, nanostructured SiC and 45.8 mmol of diamine. The reaction mass was sonicated at a frequency of 20 kHz for 20 minutes while cooling in an ice bath. Then it was stirred using a conventional mechanical stirrer. When stirring portionwise it was added 45.8 mmol of dianhydride, and then stirred for 24 hours at room temperature. The resulting mixture was cast onto a ceramic tile to obtain films using an applicator and then it was placed in a vacuum oven. The film was dried in a vacuum oven at 80 ° C for 6 hours, then at 150 ° C, 200 ° C, 250 ° C and 300 ° C for 1 hour at each temperature and then at 350 ° C for 15 minutes.
The film of composite material is obtained with a color ranging from light brown to black (depending on the composition and content of nanostructured silicon carbide).
Results and Discussion
The quality of dispersion and impregnation was determined by the photomicrographs of samples obtained by the method of scanning electron microscopy using the instrument Hitachi SU-1510 VP-SEM at a voltage of 7 kV and a working distance of 5.5 mm.
The degree of imidization is determined by the method of infrared spectroscopy using IR-Fourier transform spectrometer Vertex 70 with Raman scattering module. The degree of imidization can be determined by comparing the intensity of the absorption band (I) of the sample film and completely imidized standard film (I0) (here Kapton © of DuPont company). Normalizing factor was used to account for differences in the thickness of the films. The intensity of the absorption bands, which remain unchanged during the imidization, such as aromatic absorption bands at 1500 cm-1 (I (stand) and I0 (stand)) were used as such normalizing factor . The degree of imidization (in percentage%) is determined by the equation:
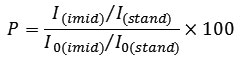
The most commonly used absorption bands of imide group: 1780cm-1 (C = O asymmetric stretching) 1380cm-1 (C-N stretching) and 725cm-1 (C = O bending). The strongest band at 1720cm-1 (C = O symmetric stretching) is also overlapped with a strong band of carboxyl group (C = O) with 1-1700cm-1 of polyamic acid. Some overlapping of specific imide bands at 1780cm-1 and 725cm-1-one is possible with anhydrides absorption bands at 1780cm-1 and 720cm-1 respectively. Bands of carboxylic acid group at 1700cm-1 (C = O) and 2800 – 3200cm-1 (-OH) and amide bands at 1660cm-1 (C = O), 1550cm-1 (C-N-H) and 3200 – 3300 cm-1( NH) that normally emerged on the broad peaks are also useful for the qualitative evaluation of the imidization process [28].
To determine the thermostability without degradation, it is necessary to plot a graph in the coordinates temperature – weight, to find the first drop of weight on the graph corresponding to the decomposition of the sample. Thermal resistance without destruction is determined by the intersection of the tangent to the graph of weight change before and after bending. Measurement is carried out using a thermal analyzer TA Instruments SDT Q 600 for simultaneous DSC/TGA/DTA analysis.
Mechanical tests were carried out on a universal testing machine LFM-20 produced by Walter + Bai AG, which provides flexural loading at a constant speed of movement of the active capture of 10 mm / min, the load measurement to a precision of less than 1% of the measured value, the ability to control sample loading rate. During testing, the voltage and the specimen elongation were recorded continuously. Tests were performed at a rate of 500 mm/min. Processing of the results was carried out using DionPro software.
Automatic recording of “load-deflection” was carried out. To measure the size of the sample it was used electronic thickness gauge with an accuracy of at most ± 0,01 mm. The tests were conducted at a temperature (23 ± 2) ° C.
Sample Preparation
To prepare the composite materials, six PI matrices were obtained, shown in Table 1. To prepare the composites with CNTs and SiC, only BPDA-ODA and PMDA-ODA matrices were used (No. 1 and 2 Table 1, respectively) for PI-CF all six were used. To prepare the composites, 3 types of fillers were used: CNT, CF and SiC. The content of fillers in composites are shown in Tables 2, 3 and 4 respectively.
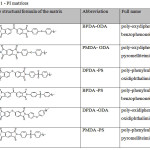 |
Table 1: PI matrices |
The infrared spectra of the films (Fig.1) were common for all composites and had absorption peaks specific to imide groups (1775 cm-1 (C=O asymmetric stretching), 1712 cm-1 (C=O symmetric stretching), 1368 cm-1 (C-N stretching), and 721 cm-1 (C=O bending). The absence of absorption bands at 1660 cm-1 corresponding to C=O amide stretching in the FTIR spectra of polyimides indicates complete imidization.
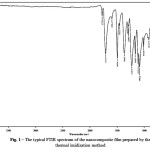 |
Figure 1: The typical FTIR spectrum of the nanocomposite film prepared by the thermal imidization method |
Morphology
Pictures of the obtained samples and photomicrographs are shown in Fig. 2. The separate CNTs (Fig. 2b) and SiC particles (Fig. 2f) are marked with red circles. The figure shows that the distribution of particles in the matrix of polyimides is uniform. So, we managed to spread polyimide evenly on the surface of carbon fibers (Fig. 2d)
 |
Figure 2: Photograph of composite material samples (a – PI- CNT film, b – a photomicrograph of PI-CNT, c – PI- Carbon fiber (fabric), d – a photomicrograph of PI-HC, e – PI- SiC film, f – photomicrograph PI- SiC.) |
Thermal Properties
Table 2 shows data on the thermostability and resistance to thermal oxidative degradation of composite materials based on carbon nanotubes (CNTs) and polyimide.
Table 2: Temperature of 5% and 10% weight losses for experimental CM samples based on carbon nanotubes and polyimide in an inert atmosphere and in air.
| No. | Matrix | Filler | Filler content, % wt. | Medium | |||
| Inert | Air | ||||||
| Td5, ºС | Td10, ºС | Td5, ºС | Td10, ºС | ||||
| 1 | PMDA -ODA | Without filler | 0 | 582,0 | 588,0 | 532,0 | 571,0 |
| 2 | Multi-walled CNT | 1,0 | 580,8 | 595,9 | 545,3 | 571,1 | |
| 3 | Multi-walled CNT | 2,0 | 576,8 | 591,1 | 575,0 | 592,2 | |
| 4 | Single-walled CNT | 1,0 | 573,6 | 588,8 | 562,9 | 583,3 | |
| 5 | Single-walled CNT | 2,0 | 577,4 | 590,1 | 564,8 | 584,1 | |
| 6 | BPDA -ODA | Without filler | 0 | 533,0 | 557,0 | 508,0 | 548,0 |
| 7 | Single-walled CNT | 1,0 | 566,0 | 585,8 | 524,7 | 561,9 | |
| 8 | Single-walled CNT | 2,0 | 552,0 | 572,9 | 528,3 | 552,1 | |
From the data of Table 2, it can be concluded, that when using the heat-resistant matrix such as PMDA-ODA, additional filler in the form of an additive CNT does not significantly increase thermostability in inert atmosphere. When using polyimide matrix less resistant to high temperature, thermostability from 533,0ºC in the initial matrix and to 566,0ºC in the matrix with 1% by weight SWCNT filler. Regardless of matrix used and the type of CNT, increasing the content of carbon nanotubes leads to an increase in resistance to thermal oxidative degradation of the composite material. Moreover, compositions based on poly-oxydiphenylene-pyromellitimide with 2% by weight of multi-walled CNTs have greater resistance to thermo-oxidative degradation.
Table 3 shows the thermostability of composite materials based on carbon fibers and polyimide. It should be noted, that the thermal resistance of the carbon fiber in an inert atmosphere (by the example of Td5) significantly exceeds 800°C.
Based on the data from Table 3, it can be concluded, that the composite materials based on carbon fiber covered of poly-oxydiphenylene-benzophenone imide have the highest heat resistance. Significantly increasing thermostability, which can be observed at a temperature of 10% of weight loss relates primarily to the small amount of polymeric binder after destruction of which carbon fibers remain only, the thermal resistance of which greatly exceeds the thermostability of the binder. The composite materials based on carbon fibers in a matrix of poly-phenylsulfone-benzophenone imide have the greatest resistance to thermo-oxidative degradation. However, these results are more defined by low content of polyimide binder and high thermal resistance of the carbon fiber. The composites based on poly-oxydiphenylene-pyromellitimide and poly-oxydiphenylene-benzophenone imide stand out among other composites with carbon fiber due to their good resistance to thermo-oxidative degradation.
Table 3: Temperature of 5% and 10% the weight losses for experimental CM samples based on carbon nanotubes and polyimide in an inert atmosphere and in air
| No. | Matrix | Filler | Filler content, % wt. | Medium | |||
| Inert | Air | ||||||
| Td5, ºС | Td10, ºС | Td5, ºС | Td10, ºС | ||||
| 1 | DPDA-ODA | Carbon fabric UT-900-PM of Argon, LLC | 0 | 531,6 | 558,0 | 517,1 | 547,4 |
| 86,8 | 528,7 | 565,5 | 530,2 | 551,2 | |||
| 2 | PMDA-ODA | 0 | 582,0 | 588,0 | 532,0 | 571,0 | |
| 79,1 | 594,8 | 624,5 | 576,9 | 601,6 | |||
| 3 | DPDA-PS | 82,3 | 480,6 | 525,0 | 516,1 | 552,9 | |
| 4 | BPDA-PS | 94,7 | 576,3 | 736,8 | 580,1 | 606,5 | |
| 5 | BPDA-ODA | 0 | 533,0 | 557,0 | 508,0 | 548,0 | |
| 80,73 | 603,4 | 761,2 | 574,2 | 602,0 | |||
| 6 | PMDA-PS | 82,8 | 565,4 | 618,7 | 572,7 | 598,6 | |
Table 4 shows data of the thermostability and the resistance to thermal oxidative degradation of composite materials based on nanostructured silicon carbide and polyimide.
Table 4: Temperature of losses of 5% and 10% of the weight of experimental CM samples based on nanostructured silicon carbide and the polyimide in an inert atmosphere and in air.
| No. | Matrix | Filler | Filler content, % wt. | Medium | |||
| Inert | Air | ||||||
| Td5, ºС | Td10, ºС | Td5, ºС | Td10, ºС | ||||
| – | PMDA -ODA | Nanostructured silicon carbide, produced by InChemSyntesis, LLC | 0,0 | 582,0 | 588,0 | 532,0 | 571,0 |
| 1 | 2,0 | 586,0 | 600,9 | 581,6 | 599,8 | ||
| 2 | 4,0 | 582,7 | 596,6 | 584,3 | 603,1 | ||
| 3 | 20,0 | 586,6 | 600,4 | 591,8 | 606,3 | ||
| – | BPDA -ODA | 0,0 | 533,0 | 557,0 | 508,0 | 548,0 | |
| 4 | 2,0 | 556,1 | 574,9 | 549,9 | 583,9 | ||
| 5 | 4,0 | 562,9 | 582,2 | 560,7 | 586,2 | ||
| 6 | 20,0 | 569,7 | 589,1 | 572,0 | 591,8 | ||
Based on the data from Table 4, it can be concluded, that when using the heat-resistant matrix, such as poly-oxydiphenylene-pyromellitimide, additional filler such as a nanostructured silicon carbide does not significantly increase thermostability under an inert atmosphere. When using the polyimide matrix less resistant to high temperature, thermostability rises from 533,0ºC in the initial matrix and to 569,7ºC in the matrix with 20% by weight of filler.
Mechanical Properties
The measured mechanical properties are different for composite films and carbon fiber-reinforced plastics. For composite films (in the case of nanostructured composites based on silicon carbide and carbon nanotubes) is used tensile stress and elongation at break. For composites with carbon fibers is used bending stress and the modulus of flexural strength.
Table 5 shows data on the mechanical properties of composite materials based on carbon nanotubes (CNTs) and polyimide (in the form of films).
Table 5: Mechanical properties of experimental CM samples based on carbon nanotubes and polyimide.
| No. | Matrix | Filler | Filler content, % wt. | Tensile stress, MPa | Elongation at break% |
| 1 | PMDA -ODA | SWCNT | 1,0 | 94 | 36 |
| 2 | SWCNT | 2,0 | 107,7 | 130,9 | |
| 3 | MWCNT | 1,0 | 102 | 28,4 | |
| 4 | MWCNT | 2,0 | 57,8 | 15,8 | |
| 5 | BPDA -ODA | SWCNT | 1,0 | 47,4 | 5,4 |
| 8 | SWCNT | 2,0 | 30 | 9,2 |
Table 6 shows data of the mechanical properties of composite materials based on carbon fiber and polyimide.
Table 6: Mechanical properties of experimental CM samples based on carbon fiber and polyimide
| No. | Matrix | Filler | Filler content, % wt. | Bending strength, MPa | Modulus of elasticity, MPa |
| 1 | DPDA -ODA | Carbon fabric УТ-900-ПМ produced by Аргон, LLC |
86,8 | 32 | 2355 |
| 2 | PMDA -ODA | 79,1 | 40 | 4458 | |
| 3 | DPDA -PS | 82,3 | 27 | 2237 | |
| 4 | BPDA -PS | 94,7 | 48 | 5587 | |
| 5 | BPDA -ODA | 80,73 | 43 | 2915 | |
| 6 | PMDA -PS | 82,8 | 35 | 2404 |
Table 7 shows data of the mechanical properties of composite materials based on nanostructured silicon carbide and polyimide.
Table 7: The mechanical properties of the experimental CM samples based on nanostructured silicon carbide and polyimide.
| No. | Matrix | Filler | Filler content, % wt. | Tensile stress, MPa* | Elongation at break%* |
| 1 | PMDA -ODA | Nanostructured silicon carbide produced by “InChemSyntesis” | 2,0 | 78,6 | 12 |
| 2 | 4,0 | 65,25 | 11,9 | ||
| 3 | 20,0 | 63,95 | 19,5 | ||
| 4 | BPDA -ODA | 2,0 | |||
| 5 | 4,0 | ||||
| 6 | 20,0 |
* – for those cases where the value of the mechanical properties is not specified and the resulting films became too fragile and broke after preparing standard samples
Conclusion
The resulting composite materials based on polyimide matrix have outstanding values of thermostability and resistance to thermo-oxidative stability, regardless of the filler content, which allow to use the received data to develop the technology of structural materials applied in the aerospace and electronics industries, especially in the areas where the materials are often exposed to extreme conditions of mechanical, electrical, radiation and other stresses.
Acknowledgement
Applied researches were carried out with the financial support of the state represented by the Russian Ministry of Education under the Grant Agreement No.14.625.21.0003 of August 25, 2014. (Unique identifier of applied scientific researches (project) RFMEFI62514X0003).
References
- Berlin A.A. Polymeric composite materials: structure, properties, technology. 2014, 4-th ed. SP, «Proffessya», 592.
- Vshivkov, S.A. Lection materials2011.A. M. Gorky Ural.State University, 70.
- Kryzhanovsky V.K., Kerber M.L., Burlov V.V., Panimatchenko A.D. Manufacturing of polymeric materials.2004, SP, Professiya, 464.
- Kryzhanovsky V.K., Nikolaev A. F. Technology of polymeric materials.2011, SP, Professiya 536.
- Zou H, Wu S, Shen J. Chem. Rev.2008,108, 3893–3957.
- Wang P.J., Lin C.H., Chang S.L., Shih S.J. Polym. Chem.2012,3, 2867–2874.
- Zhu J., Wei S., Haldolaarachchige N., Young D.P., Guo Z. J. Phys. Chem. C.2011,115, 15304–15310.
- Kobayashi Y., Katakami H., Mine E., Nagao D., Konno M., Liz-Marzan L.M. J. Colloid. Interface. Sci. 2005, 283, 392–396.
- Mavinakuli P., Wei S., Wang Q., Karki A.B., Dhage S., Wang Z., et al. J. Phys. Chem. P art C.2010, 114, 3874–3882.
- Agag T., Koga T., Takeichi T. Polymer.2001, 42, 3399–3408.
- Yegorov A.S, Ivanov V.S, Antipov A.V, Wozniak A.I, Tcarkova K.V. Orient. J. Chem. 2015.31(3), 1269-1275.
- Wozniak A.I., Ivanov V.S., Retivov V.M., Yegorov A.S. Oriental Journal of Chemistrу.2015,31(3), 1545-1550.
- Wozniak A.I., Yegorov A.S., Ivanov V.S., Igumnov S. M., Tcarkova K.V. Journal of Fluorine Chemistry.2015, 180, 45-54.
- MichailinYu.AThermostable polymers and polymeric materials.2006, SP, Professiya, 624.
- Wang J.-Y., Yang S.-Y., Huang Y.-L., Tien H.-W., Chin W.-K., Ma C.-C.J. Mater. Chem.2011,21, 13569-13575.
- Geckeler K. E. Trends Polym.Sci.1994, 2, 355.
- Martin N., Giacalone F., Prato M. Fullerene Polymers: Synthesis, Properties and Applications. 2009, Wiley-VCH.N.-Y, 210.
- Shinoda Y., Shiota I., Ishida Y., Ogasawara T., Yokota R. Adv. Sci. Tech.2006.51, 75-80.
- Seo Y., JikeiМ.,KakimotoМ., Imai Y. High Perform. Polym.1997, 9, 205–214
- Ishikawa T. Comp. Sci. and Tech.1994,51, 135-144
- Naito K. J. Mater. Sci.2013,48, 4163-4176
- Clair T.L.S. Polyimides composites based on asymmetric dianhydrides. International sample symposium.2009, Baltimore, 115-120.
- Clair A.K.S.; Clair T.L.S. U. S. Patent1986, 4603061, NASA.
- Simpson J., Ounaies Z., Fay C. Mat. Res. Soc. Symp. Proc.1997, 459, 53-55.
- Park Ch., Ounaies Z., Watson K.A., Crooks R. E., Smith J., LowtherSh.E., Connell J.W., Siochi E.J., Harrison J.S., Clair T.L.St. Chem. Phys. Lett.2002,364, 303-308.
- Lim J., Shin D. G., Yeo H., Goh M., Ku B.-Ch., Yang Ch.-M., Lee D.S., Hwang J.-Y., Park B., You N.-H. J. Poly. Sci. B: Poly. Phys.2014, 52, 960-966.
- Yegorov A.S. Ivanov V.S. Wozniak A.I. Biosciences Biotechnology Research Asia2014,11(3), 1765-1779.
- Layus L.A., Bessonov M.I., Kallistova E.V., Adrova N.A., Florinskiy F.S. Visokomolekularniyesoedineniya.1967,A9(10), 2185-2193.

This work is licensed under a Creative Commons Attribution 4.0 International License.









